水杨醛活化Co(II)催化吲哚与烯烃的C-H氧化烯化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以低成本的Co(NO3)2·6H2O为催化剂,Mn(OAc)2为氧化剂,采用配体促进了吲哚和烯烃的氧化脱氢C-H烯化反应。设计和选择电独特的甲基取代水杨醛作为配体是实现这种转化的关键。该方法可以将吲哚主链引入多种生物活性分子,如布洛芬、萘普生和雌二醇,用于后期合成修饰,这在发现含有吲哚基序的药物分子方面具有潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
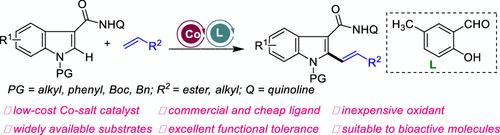
Salicylaldehyde-Enabled Co(II)-Catalyzed Oxidative C–H Alkenylation of Indoles with Olefins
A ligand-promoted oxidative dehydrogenation C–H alkenylation of indoles and olefins was achieved using commercial and low-cost Co(NO3)2·6H2O as a catalyst and Mn(OAc)2 as an oxidant. The design and selection of electrically unique methyl-substituted salicylaldehyde as a ligand is the key to achieve this transformation. This protocol can introduce an indole backbone into diverse bioactive molecules such as ibuprofen, naproxen, and Estrol for late-stage synthetic modification, which has potential applications in the discovery of drug molecules containing an indole motif.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


