丝光沸石骨架外阳离子的电负性与CO2气体吸附能力的关系
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
合成丝光沸石被广泛用作分子筛、吸附剂和催化剂。为了增强这些功能,了解沸石的离子交换特性和阳离子交换位点是至关重要的。在本研究中,我们利用同步x射线粉末衍射分析了环境条件下完全交换Cs、Sr、Cd和pb的丝光沸石的结构变化。Rietveld结构细化表明,Cs+阳离子由于其低电负性和水合能,主要位于8元环(8MR)附近。相比之下,二价阳离子如Sr2+和Cd2+阳离子的水合能比一价阳离子高,它们以水合离子的形式沿c轴(12MRc)出现在12元环的中心。由于Pb2+离子的电负性高于框架原子,因此对框架氧原子的电子云具有很强的亲和力,这使得它们靠近12MRc的壁。观察到的框架外阳离子位置的差异归因于静电和水合作用。此外,根据可交换阳离子的类型和位置对CO2吸附能力进行了评价。研究结果表明,CO2吸附能力的提高与能与CO2有效相互作用的阳离子数量有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
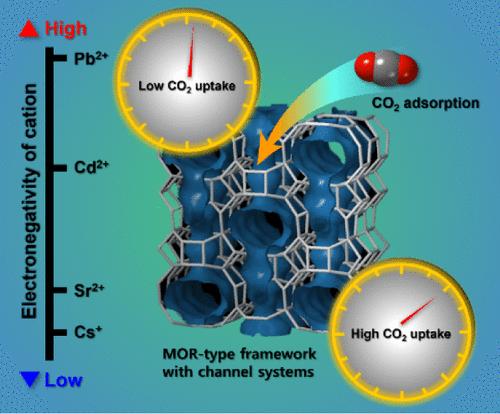
Relationship Between Electronegativity of the Extra-Framework Cations and Adsorption Capacity for CO2 Gas on Mordenite Framework
Synthetic mordenite is widely used as a molecular sieve, adsorbent, and catalyst. To enhance these functionalities, it is crucial to understand the ion-exchange properties and cation-exchange sites of the zeolite. In this study, we analyzed the structural changes in fully Cs-, Sr-, Cd-, and Pb-exchanged mordenite by using synchrotron X-ray powder diffraction under ambient conditions. Rietveld structure refinement revealed that the Cs+ cation is predominantly located near the 8-membered ring (8MR) due to its low electronegativity and hydration energy. In contrast, divalent cations such as Sr2+ and Cd2+ cations, with higher hydration energies compared to monovalent cations, are present as hydrated ions at the center of the 12-membered ring along the c-axis (12MRc). Pb2+ ions, due to their higher electronegativity than the framework atoms, exhibit a strong affinity for the electron cloud of framework oxygen atoms, which positions them close to the wall of the 12MRc. The observed differences in the locations of the extra-framework cations are attributed to electrostatic and hydration effects. Furthermore, the CO2 adsorption capacity was assessed based on the type and site of exchangeable cations. The findings indicate that an increase in the CO2 adsorption capacity correlates with the number of cations that can effectively interact with CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


