新的PD-L1/VISTA双重抑制剂作为潜在的免疫治疗药物
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
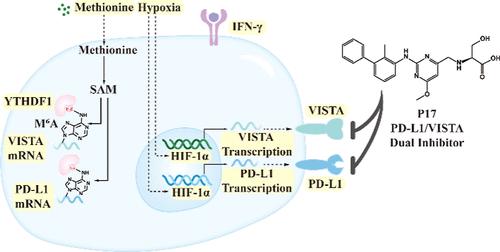
抑制免疫检查点蛋白的活性以重新激发免疫细胞的抗肿瘤活性已成为一种关键策略。PD-L1和VISTA作为控制免疫调节的关键蛋白,在缺氧等条件下同时上调。通过合理的药物设计过程,确定了PD-L1和VISTA的双靶点抑制剂P17。这种抑制剂在蛋白和细胞水平上阻断PD-L1和VISTA的信号通路,从而重新激活T细胞的抗肿瘤功能。P17在可用药性和安全性评价方面表现出令人鼓舞的属性。值得注意的是,在体内实验中,与相同剂量的单靶点抑制剂相比,P17显示出更好的抗肿瘤功效。更重要的是,P17显著增强了免疫细胞的浸润。本研究不仅验证了针对PD-L1和VISTA的双靶点抑制剂策略的有效性,而且还确定了P17是一个具有显著治疗潜力的有希望的候选分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel PD-L1/VISTA Dual Inhibitor as Potential Immunotherapy Agents
Inhibiting the activity of immune checkpoint proteins to reignite the antitumor activity of immune cells has emerged as a pivotal strategy. PD-L1 and VISTA, as critical proteins governing immune regulation, are concurrently upregulated under conditions such as hypoxia. Through a rational drug design process, P17, a dual-target inhibitor for PD-L1 and VISTA is identified. This inhibitor blocks the signaling pathways of both PD-L1 and VISTA at the protein and cellular levels, thereby reactivating the antitumor function of T cells. P17 displays encouraging attributes in terms of druggability and safety assessments. Notably, P17 demonstrates superior antitumor efficacy compared to single-target inhibitors at equivalent doses in in vivo experiments. More crucially, P17 significantly enhances the infiltration of immune cells. This study not only validates the effectiveness of a dual-target inhibitor strategy against PD-L1 and VISTA, but also identifies P17 as a promising candidate molecule with significant therapeutic potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


