光氧化催化2-苯基苯甲酸与n -氨基吡啶盐的氨基内酯化反应制备4-磺胺-3,4-二氢异香豆素
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以n -氨基吡啶盐为氨基自由基前体,实现了光氧化催化不饱和羧酸的氨基内酯化反应。该转化条件温和,底物范围广,为构建广泛的4-磺氨基3,4-二氢异香豆素提供了一种有效的方法。机理研究表明,该反应是通过独特的n -氨基吡啶盐促进2-苯基苯甲酸的亲电胺化进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
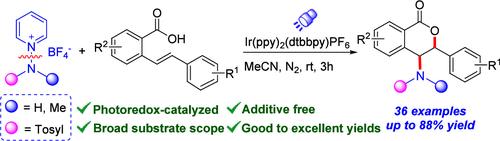
Photoredox-Catalyzed Aminolactonization of 2-Styrylbenzoic Acids with N-Aminopyridinium Salts to Access 4-Sulfonamino-3,4-dihydroisocoumarins
A photoredox-catalyzed aminolactonization of unsaturated carboxylic acids was achieved using N-aminopyridinium salts as the amino radical precursor. The transformation features mild conditions and a remarkably broad substrate scope, offering an efficient approach to construct a wide range of 4-sulfonamino 3,4-dihydroisocoumarins. Mechanistic studies indicate that the reaction proceeds via a distinctive N-aminopyridinium salt-promoted electrophilic amination of 2-styrylbenzoic acids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


