TKK130是一种3-羟基丙胺(HPA),体内抗疟活性强,耐药屏障高
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
疟疾继续对流行地区的人口造成重大负担,需要创新的治疗方案。本文报道了新型3-羟基丙脒(HPA) 2 (TKK130)的合成和临床前评价,该药物对药物敏感和耐药的恶性疟原虫具有良好的体外抗疟原虫活性。此外,在多种人类细胞系中,该化合物表现出无细胞毒性和良好的寄生虫选择性。该化合物抑制合成血色素(β-血红素)的形成,IC50值低于氯喹(CQ),体外活性与速效抗疟药双氢青蒿素相当。此外,选择研究显示抗性发展的倾向非常低。根据最初的体内药代动力学快照数据,2 (TKK130)具有持久的线性药代动力学特征。在体内,这种新型HPA在伯格氏疟原虫模型中表现出治疗活性,在p中表现出强效活性。口服给药后恶性疟原虫SCID小鼠模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
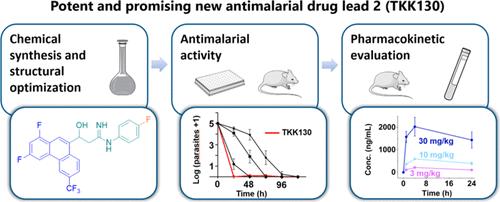
TKK130 is a 3-Hydroxy-Propanamidine (HPA) with Potent Antimalarial In Vivo Activity and a High Barrier to Resistance
Malaria continues to pose a significant burden on populations in endemic areas and requires innovative treatment options. Here, we report the synthesis and preclinical evaluation of the novel 3-hydroxypropanamidine (HPA) 2 (TKK130), which shows excellent antiplasmodial in vitro activity against drug-sensitive and -resistant Plasmodium falciparum strains. Moreover, in various human cell lines, the compound shows no cytotoxicity and excellent parasite selectivity. The compound inhibits synthetic hemozoin (β-hematin) formation, with IC50 values lower than chloroquine (CQ), and its in vitro rate of activity is comparable with the fast-acting antimalarial drug dihydroartemisinin. Furthermore, selection studies reveal a very low propensity for resistance development. Based on initial in vivo pharmacokinetic snapshot data, 2 (TKK130) has a long-lasting, linear pharmacokinetic profile. In vivo, this novel HPA exhibits curative activity in the Plasmodium bergheimouse model and potent activity in theP. falciparum SCID mouse model after oral administration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


