糖尿病肾病患者肾小球醛缩酶B的缺失通过激活Akt/GSK/β-连环蛋白轴促进肾纤维化
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
目的糖尿病肾病(DN)是终末期肾病(ESRD)最重要的催化剂,其发病机制复杂且多面性。本研究旨在分析肾小球醛缩酶B (glomer小球醛缩酶B, ALDOB)在肾病进展中的水平及其非代谢作用。方法对50例DN患者和25例对照组进行肾小球蛋白质组学和转录组学分析。采用人肾活检、培养足细胞和小鼠模型研究ALDOB水平和功能。结果在dn影响的肾小球以及暴露于炎性细胞因子的人和鼠足细胞中,saldob被强烈下调。在糖尿病小鼠模型中,ALDOB减少会增加足细胞损伤,而腺病毒介导的ALDOB过表达会显著减轻肾损伤。在机制上,ALDOB的减少触发足细胞内Akt/GSK/β-catenin信号级联。结论肾小球ALDOB在防止足细胞损伤和肾纤维化方面具有新的非代谢作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
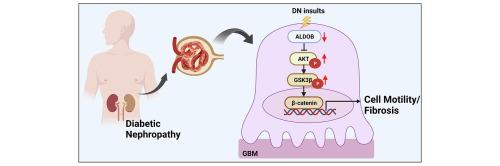
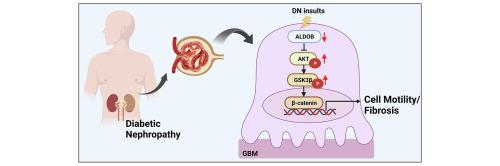
Loss of glomerular aldolase B in diabetic nephropathy promotes renal fibrosis via activating Akt/GSK/β-catenin axis
Objective
Diabetic nephropathy (DN), characterized by a complex and multifaceted pathogenesis, stands as the foremost catalyst behind end-stage renal disease (ESRD). This study aims to analyze the level and non-metabolic role of glomerular aldolase B (ALDOB) in DN progression.
Methods
Glomerular proteomics and transcriptome are analyzed from 50 DN patients and 25 controls, respectively. Human kidney biopsy, cultured podocytes and mouse models are employed to study ALDOB levels and function.
Results
ALDOB is strongly downregulated in DN-affected glomeruli, as well as in human and murine podocytes exposed to inflammatory cytokines. ALDOB reduction increases podocyte injury, while adenovirus-mediated ALDOB overexpression leads to substantial alleviation of renal injuries in a diabetic mouse model. Mechanistically, ALDOB reduction triggers the Akt/GSK/β-catenin signaling cascade within podocytes.
Conclusion
Our findings reveal a novel non-metabolic role of glomerular ALDOB in protecting against podocyte injury and renal fibrosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


