靶向APJ驱动bniip3 - pink1 - parkin诱导的线粒体自噬,改善全身性炎症性骨丢失
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
炎症性疾病,如糖尿病、类风湿关节炎和炎症性肠病,会导致全身免疫微环境紊乱,导致骨质流失,然而特异性受体在炎症性骨质流失中调节这一过程的机制尚不清楚。Apelin受体作为一种g蛋白偶联受体,在炎症和免疫微环境的调控中起着至关重要的作用。然而,控制其在炎症性骨质流失中的作用的确切机制仍然不完全清楚。目的探讨APJ调控巨噬细胞极化减轻炎性骨质流失的机制。方法采用聚多糖诱导小鼠全身性炎症性骨质流失模型,探讨骨质流失与破骨细胞活化、巨噬细胞极化和APJ的关系。在体外研究中,我们利用骨髓源性巨噬细胞和siRNA来阐明APJ对免疫微环境和破骨细胞分化的调节作用,同时利用高通量测序来揭示APJ调节巨噬细胞极化的潜在机制。结果本研究建立了APJ与系统性炎性骨质丢失小鼠巨噬细胞M1极化之间的联系。激活APJ可有效减轻巨噬细胞M1极化,抑制破骨细胞过度活化,减轻全身性炎症性骨质流失。体外高通量测序分析显示,APJ调节巨噬细胞极化,与线粒体自噬和nod样受体信号通路有关,APJ激活后AMPK和MAPK信号通路也参与了信号转导。随后的实验证实,APJ主要通过调节AMPK/BNIP3/PINK1/PARKIN轴来增强线粒体自噬,减少活性氧的积累,从而抑制巨噬细胞M1极化的激活和破骨细胞的发生。结论本研究阐明了APJ调控巨噬细胞极化的潜在机制,为解决炎症性骨质流失提供了新的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
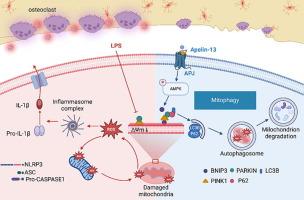
Targeting APJ drives BNIP3-PINK1-PARKIN induced mitophagy and improves systemic inflammatory bone loss
Introduction
Inflammatory diseases, such as diabetes mellitus, rheumatoid arthritis, and inflammatory bowel disease, lead to systemic immune microenvironment disturbances, contributing to bone loss, yet the mechanisms by which specific receptors regulate this process in inflammatory bone loss remain poorly understood. As a G-protein-coupled receptor, the Apelin receptor plays a crucial role in the regulation of inflammation and immune microenvironment. However, the precise mechanisms governing its role in inflammatory bone loss remain incompletely understood.
Objective
This study aims to investigate how APJ regulates macrophage polarization to mitigate inflammatory bone loss.
Methods
Lipopolysaccharide induced systemic inflammatory bone loss model in mice was used to explore the relationship between bone loss and osteoclast activation, macrophage polarization and APJ. In vitro studies, Bone marrow derived macrophages and siRNA were used to elucidate the regulatory influence of APJ on the immune microenvironment and osteoclast differentiation, while high-throughput sequencing is leveraged to uncover the underlying mechanisms through which APJ modulates macrophage polarization.
Results
Our study established a link between APJ and macrophage M1 polarization in systemic inflammatory bone loss mice. The activation of APJ effectively mitigated M1 polarization in macrophages, suppressed excessive osteoclast activation, and alleviated systemic inflammatory bone loss. In vitro high-throughput sequencing analysis revealed that APJ modulates macrophage polarization, linking to mitochondrial autophagy and the NOD-like receptor signaling pathway and the involvement of the AMPK and MAPK signaling pathways in signal transduction after APJ activation was also suggested. Subsequent experiments substantiated that APJ predominantly enhances mitophagy and diminishes the accumulation of reactive oxygen species by regulating the AMPK/BNIP3/PINK1/PARKIN axis, thereby suppressing the activation of macrophage M1 polarization and osteoclastogenesis.
Conclusion
This study elucidated the underlying mechanism by which APJ modulates macrophage polarization, thereby proposing a new therapeutic target for addressing inflammatory bone loss.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


