添加二氧化二甲醚增强锂硫电池反应动力学及稳定锂沉积
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
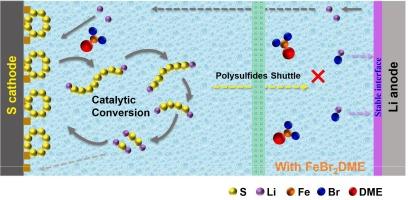
锂硫(li -硫)电池具有较高的理论比容量和能量密度,有望改变储能技术。然而,诸如多硫化物穿梭和锂阳极不稳定性等挑战阻碍了它们的商业化。本研究介绍了一种新型电解质添加剂——溴化铁二甲氧基乙烷(FeBr2DME),它能催化多硫化物转化,提高反应动力学,稳定锂沉积。在Li-Li对称电池中,FeBr2DME可以在1 mA cm−2下稳定循环1000 h。锂铜电池在200次循环中保持了高库仑效率(~ 99 %)。在电解质中加入0.5 mM的FeBr2DME,在4C下的比放电容量为519.3 mAh g−1,在0.2C下循环100次后的容量保持率为90.3 %。即使在高硫负荷(3.1 mg cm−2)和低电解质/硫比(5 μL mg−1)的情况下,电池在0.2C下循环100次后的放电比容量为425.8 mAh g−1,表现出优异的循环稳定性。这些发现突出了FeBr2DME作为双功能电解质添加剂的潜力,为Li-S电池的关键挑战提供了可行的解决方案,并为其商业可行性铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing reaction kinetics and stabilizing lithium deposition in lithium-sulfur batteries with an FeBr2DME additive
Lithium-sulfur (Li-S) batteries, with their high theoretical specific capacity and energy density, hold the promise of transforming energy storage. However, challenges such as polysulfide shuttling and lithium anode instability hinder their commercialization. This study introduces an innovative electrolyte additive, iron bromide dimethoxyethane (FeBr2DME), which catalyzes polysulfide conversion, enhances reaction kinetics, and stabilizes lithium deposition. In Li-Li symmetric cells, FeBr2DME enabled stable cycling for 1000 h at 1 mA cm−2. Li-Cu cells maintained high coulombic efficiencies (∼99 %) over 200 cycles. Adding 0.5 mM FeBr2DME to the electrolyte resulted in a specific discharge capacity of 519.3 mAh g−1 at 4C, with 90.3 % capacity retention after 100 cycles at 0.2C. Even with high sulfur loading (3.1 mg cm−2) and a low electrolyte/sulfur ratio (5 μL mg−1), the batteries achieved a discharge specific capacity of 425.8 mAh g−1 after 100 cycles at 0.2C, showcasing exceptional cycling stability. These findings highlight FeBr2DME’s potential as a dual-function electrolyte additive, offering a viable solution to key challenges in Li-S batteries and paving the way for their commercial viability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


