三聚氰胺驱动的溶剂化效应促进了铂催化剂上的氧还原:机器学习辅助自由能计算
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
用三聚氰胺等有机化合物修饰铂表面,提高了氧还原反应活性和催化剂耐久性。通过利用热力学积分和有限温度分子动力学的第一性原理自由能计算,通过机器学习力场的增强,以有效采样纳秒级的界面水波动,并结合修正来精确地重现第一性原理自由能,我们证明了三聚氰胺破坏OH吸附的稳定性,促进了它们的去除并增强了催化活性。不像合金,氢氧根的不稳定是由电子结构和表面应变的变化驱动的,三聚氰胺破坏氢氧根和界面水之间的氢键。结构和振动分析表明,三聚氰胺改变了水溶液的溶剂化结构,这在径向分布函数的改变和O-H拉伸振动的蓝移中表现得很明显。这些发现表明,操纵有机化合物的界面溶剂化可能是一种很有前途的方法,可以在不影响耐久性的情况下提高催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
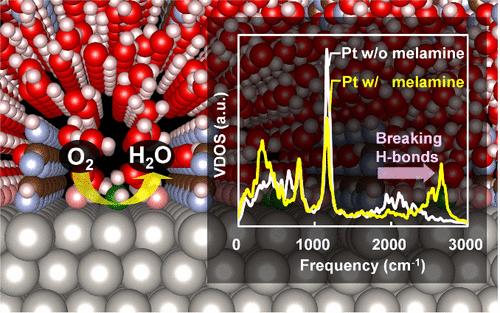
The Melamine-Driven Solvation Effect Promotes Oxygen Reduction on a Platinum Catalyst: Machine Learning-Aided Free Energy Calculations
The modification of Pt surfaces with organic compounds like melamine enhances oxygen reduction reaction activity and catalyst durability. Through first-principles free energy calculations utilizing thermodynamic integration and finite-temperature molecular dynamics, enhanced by machine learning force fields for efficient sampling of nanosecond-scale interfacial water fluctuations and incorporating corrections to accurately reproduce first-principles free energies, we demonstrate that melamine destabilizes OH adsorbates, facilitating their removal and enhancing catalytic activity. Unlike alloys, where OH destabilization is driven by changes in electronic structure and surface strain, melamine disrupts hydrogen bonding between OH and interfacial water. Structural and vibrational analyses reveal that melamine alters the water solvation structure, which is evident in modified radial distribution functions and a blue shift in the O–H stretching vibrations. These findings indicate that manipulating interfacial solvation with organic compounds could be a promising approach to enhance catalytic activity without compromising durability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


