植物性食品中双酚A及其类似物的浓度、成分概况和体外硅基混合物风险评估
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
双酚A (BPA)与结构相似的类似物的替代引起了人们的关注,因为它们具有类似的雌激素活性。考虑到植物性食品的高消费,评估此类饮食来源中的双酚类(bp)带来的风险至关重要。然而,有限的BP类似物暴露和动物毒理学数据阻碍了全面的风险评估。本研究调查了台湾23种植物性食物中的16种bp,并估计了它们在不同年龄组的饮食暴露量。采用高通量毒物动力学模型将体外ToxCast雌激素受体(ER)生物活性浓度转化为人体等效出发点(pod),并将其与动物研究得出的pod进行比较,并应用于基于常见ER途径的暴露边际评估混合风险。总共检测到7种bp,大多数样品(85.9 %)含有可检测浓度。总7个基点(∑7 bp)的浓度范围从0.06 ±0.11 26.60 ng / g ±72.18 ng / g, BPA是最主要的(63 %的∑7 bp浓度),其次是双酚S(19 %)和4,4-bisphenol F(13 %)。在体外,硅衍生的豆荚与体内动物衍生的豆荚具有相当的保护作用,甚至比体内动物衍生的豆荚更具保护作用。对于大多数人群来说,从植物性食物中同时接触多种bp通常不会引起内质网途径紊乱的风险,尽管不能排除最坏情况下的潜在担忧。这项研究促进了对与BP混合物相关的饮食风险的理解,并说明了体外硅片方法评估环境污染物对人类健康风险的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
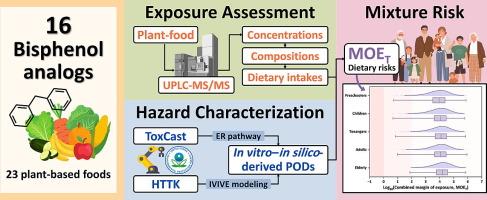

Concentrations, composition profiles, and in vitro–in silico-based mixture risk assessment of bisphenol A and its analogs in plant-based foods
The substitution of bisphenol A (BPA) with structurally similar analogs has raised concerns due to their comparable estrogenic activities. Considering the high consumption of plant-based foods, assessing the risks posed by bisphenols (BPs) in such dietary sources is essential. However, limited exposure and animal toxicological data on BP analogs hinder comprehensive risk assessments. This study investigated 16 BPs in 23 plant-based foods from Taiwan and estimated their dietary exposure across age groups. High-throughput toxicokinetic modeling was used to convert in vitro ToxCast estrogen receptor (ER) bioactive concentrations into human-equivalent points of departure (PODs), which were compared to PODs derived from animal studies and applied to assess mixture risks through the margin of exposure based on the common ER pathway. In total, 7 BPs were detected, and most samples (85.9 %) contained detectable concentrations. Total concentrations of the 7 BPs (∑7BP) ranged from 0.06 ± 0.11 ng/g to 26.60 ± 72.18 ng/g, with BPA being the most predominant (63 % of the mean ∑7BP concentrations), followed by bisphenol S (19 %) and 4,4-bisphenol F (13 %). In vitro–in silico-derived PODs were comparable to or even more protective than in vivo animal-derived PODs. For most population groups, combined exposure to multiple BPs from plant-based foods is generally not a risk concern for ER pathway perturbation, although potential concerns in worst-case scenarios cannot be excluded. This study advances the understanding of the dietary risks associated with BP mixtures and illustrates the potential of in vitro–in silico approaches for assessing human health risks from environmental contaminants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


