揭示草甘膦在大肠杆菌中的新蛋白相互作用伙伴
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
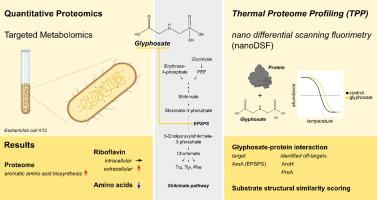
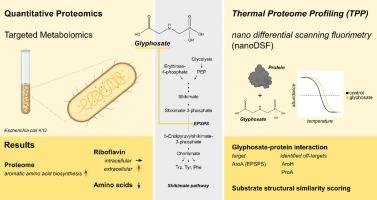
尽管关于草甘膦的安全使用存在种种争议,但草甘膦仍然是除草剂产品中应用最广泛的活性成分,欧盟将在2033年之前重新批准使用草甘膦。由于草甘膦的使用方式,非目标生物通常暴露于草甘膦,其更广泛的环境和生物影响仍在调查中。草甘膦与磷酸烯醇丙酮酸酯(PEP)具有结构相似性,从而竞争性地抑制5-烯醇丙酮酸莽草酸-3-磷酸合成酶(EPSPS),该合成酶在植物、真菌、细菌和古菌中合成芳香氨基酸至关重要。大多数微生物,包括肠道细菌大肠杆菌(E. coli),都具有对草甘膦敏感的I类EPSPS,这使得它们很容易受到草甘膦的影响。然而,关于草甘膦与其他细菌蛋白质的相互作用或其在蛋白质组水平上的更广泛的作用模式知之甚少。在这里,我们采用定量蛋白质组学和热蛋白质组分析(TPP)方法来鉴定大肠杆菌蛋白质组中草甘膦的新蛋白质结合伙伴。草甘膦暴露显著改变氨基酸合成途径。莽草酸途径蛋白丰度增加,提示有代偿机制。草甘膦暴露后细胞外核黄素浓度升高,而细胞内水平保持稳定。除了目标酶EPSPS,热蛋白质组分析表明草甘膦对某些蛋白质的热稳定性有影响,包括AroH和ProA,表明相互作用。与PEP和草甘膦在EPSPS上的竞争性结合类似,AroH和ProA与除草剂相互作用的一个原因可能是它们的底物与草甘膦之间的结构高度相似。总的来说,草甘膦诱导大肠杆菌代谢紊乱,超出其主要目标,从而为草甘膦对微生物系统的更广泛影响提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Revealing novel protein interaction partners of glyphosate in Escherichia coli
Despite all debates about its safe use, glyphosate remains the most widely applied active ingredient in herbicide products, with renewed approval in the European Union until 2033. Non-target organisms are commonly exposed to glyphosate as a matter of its mode of application, with its broader environmental and biological impacts remaining under investigation. Glyphosate displays structural similarity to phosphoenolpyruvate (PEP), thereby competitively inhibiting the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), crucial for the synthesis of aromatic amino acids in plants, fungi, bacteria, and archaea. Most microbes, including the gut bacterium Escherichia coli (E. coli), possess a glyphosate-sensitive class I EPSPS, making them vulnerable to glyphosate’s effects. Yet, little is known about glyphosate’s interactions with other bacterial proteins or its broader modes of action at the proteome level. Here, we employed a quantitative proteomics and thermal proteome profiling (TPP) approach to identify novel protein binding partners of glyphosate in the E. coli proteome. Glyphosate exposure significantly altered amino acid synthesizing pathways. The abundance of shikimate pathway proteins was increased, suggesting a compensatory mechanism. Extracellular riboflavin concentrations were elevated upon glyphosate exposure, while intracellular levels remained stable. Beyond the target enzyme EPSPS, thermal proteome profiling indicated an effect of glyphosate on the thermal stability of certain proteins, including AroH and ProA, indicating interactions. Similar to the competitive binding between PEP and glyphosate at EPSPS, one reason for the interaction of AroH and ProA with the herbicide could be a high structural similarity between their substrates and glyphosate. Overall, glyphosate induced metabolic disturbances in E. coli, extending beyond its primary target, thereby providing new insights into glyphosate’s broader impact on microbial systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


