聚乙烯微塑料暴露对人类和小鼠卵母细胞质量有不利影响
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
微塑料(MPs)是普遍存在的环境污染物,导致人类不可避免地接触。本研究确定了卵泡液中的MPs,并研究了特异性MPs及其对卵母细胞不利影响的机制。采用拉曼显微光谱法测定44例接受辅助生殖技术的不孕妇女卵泡液中的MPs。通过非靶向代谢组学分析卵泡液中的差异代谢物。雌性小鼠暴露于聚乙烯(PE)以验证人类的发现。MPs,尤其是PE在人卵泡液中检出率最高(86.4 %),与受精率呈负相关(r = -0.407,P = 0.007)。增高的PE水平改变了主要参与代谢途径、铁下垂和卵巢类固醇生成的代谢物。在小鼠中,PE暴露显著减少了取卵细胞的数量(31.5 vs 36.3, P <;P < 0.05)和受精率(70.8 % vs. 85.2 %;0.001),同时增加了劣质卵母细胞的比例(28.2 % vs. 16.5 %,P <;0.001)和活性氧(ROS)的产生。RNA测序显示,pe暴露组炎症相关基因(Il10ra、Il1a、Il33、Tnfaip8l2和Tnfrsf1b)显著上调。综上所述,PE暴露可能通过破坏卵泡液代谢、提高炎症相关基因表达和增加卵母细胞中ROS的产生而损害卵母细胞质量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
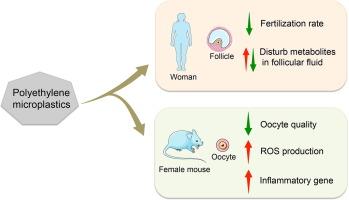

Polyethylene microplastic exposure adversely affects oocyte quality in human and mouse
Microplastics (MPs) are pervasive environmental contaminants, resulting in unavoidable human exposure. This study identified MPs in follicular fluid and investigated the specific MPs and mechanisms that adversely affect oocytes. MPs in the follicular fluid of 44 infertile women undergoing assisted reproductive technology were measured using Raman microspectroscopy. Differential metabolites in follicular fluid were analyzed via untargeted metabolomics. Female mice were exposed to polyethylene (PE) to validate human findings. MPs, particularly PE, exhibited the highest detection rate (86.4 %) in human follicular fluid and showed a negative correlation with fertilization rates (r = -0.407, P = 0.007). Elevated PE levels altered metabolites primarily involved in metabolic pathways, ferroptosis, and ovarian steroidogenesis. In mice, PE exposure significantly reduced the number of retrieved oocytes (31.5 vs. 36.3, P < 0.05) and fertilization rate (70.8 % vs. 85.2 %, P < 0.001), while increasing the proportion of poor-quality oocytes (28.2 % vs. 16.5 %, P < 0.001) and reactive oxygen species (ROS) production compared to controls. RNA sequencing indicated significant upregulation of inflammation-related genes (Il10ra, Il1a, Il33, Tnfaip8l2, and Tnfrsf1b) in the PE-exposed group. In conclusion, PE exposure impairs oocyte quality possibly by disrupting follicular fluid metabolism, elevating inflammation-related gene expression, and increasing ROS production in oocytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


