铜增强CaTiO3单电子氧还原压电催化生产H2O2
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
压电催化生产H2O2是一种新型的、环保的H2O2生产方法,目前正在开发许多压电催化剂。但它们都存在贵金属作为助催化剂、催化效率低的缺点。本文成功制备了CaTiO3,并负载了非贵金属CoP (CoP/CaTiO3)用于压电催化生产H2O2。5% CoP/CaTiO3压电催化剂的H2O2产率达到9657.3 μmol L-1 g-1,是单组分CaTiO3的1.64倍,且经过5次循环后性能保持稳定。本研究利用压电响应力显微镜直接展示了CoP/CaTiO3的压电响应。在超声条件下,CoP/CaTiO3发生极化,产生电子(e -)和空穴(h+)。e-与O2发生两步单电子反应生成H2O2。由于CoP的导带位置低于CaTiO3,这种电位差促进了e -从CaTiO3向CoP的迁移。这种迁移过程有效地增强了e -和h+的空间分离,从而显著提高了压电催化性能。本研究通过大量生产催化剂的方法合成了一种无毒、廉价的新型压电催化材料,为提高压电催化活性提供了策略,对H2O2的绿色工业生产起到了关键的推动作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
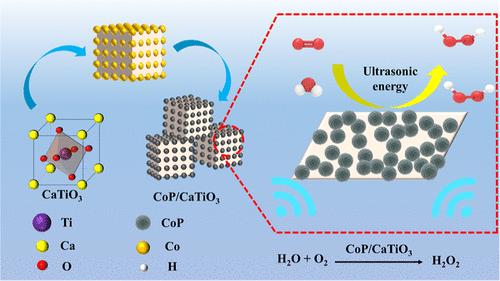
CoP-Enhanced CaTiO3 Single-Electron Oxygen Reduction Piezoelectric Catalysis for H2O2 Production
Piezoelectric catalytic production of H2O2 is a novel and environmentally friendly H2O2 production method, and many piezoelectric catalysts are currently being developed. However, all of them have the disadvantages of precious metals as cocatalysts and low catalytic efficiency. Herein, CaTiO3 was successfully prepared and loaded with the nonprecious metal CoP (CoP/CaTiO3) for piezoelectric catalytic production of H2O2. The yield of H2O2 produced by 5% CoP/CaTiO3 piezoelectric catalysis reached 9657.3 μmol L–1 g–1, which was 1.64 times than that of single-component CaTiO3, and furthermore, the performance remained stable after five cycles. This study directly demonstrates the piezoelectric response of CoP/CaTiO3 by using Piezoresponse Force Microscopy. Under ultrasonic conditions, CoP/CaTiO3 undergoes polarization, resulting in the generation of electrons (e–) and holes (h+). The e– undergoes a two-step single-electron reaction with O2 to form H2O2. Because the conduction band position of CoP is lower than that of CaTiO3, this potential difference promotes the migration of e– from CaTiO3 to CoP. This migration process effectively enhances the spatial separation of e– and h+, which significantly improves the piezoelectric catalytic performance. In this work, a nontoxic and inexpensive new piezoelectric catalytic material was synthesized by a method that can produce catalysts in large quantities, and a strategy was provided to improve the piezoelectric catalytic activity, which played a key role in promoting the green industrial production of H2O2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


