通过折叠体螺旋模拟和双硫醚钉接的p53-hDM2相互作用细胞渗透性肽抑制剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
螺旋折叠体与α-多肽结合可产生具有降低肽特性和增强抗蛋白水解能力的α-螺旋模拟物。之前,我们设计了一个复制p53 n端α-螺旋结构域的杂化肽-寡脲序列,以实现与hDM2的高亲和力结合。在这里,我们通过将折叠体方法与侧链交联相结合来进一步推进这一策略,以创建能够有效地参与细胞内靶标的更受限的细胞渗透性抑制剂。从折叠体- hdm2复合物的晶体结构出发,我们确定了适合钉接的特定位点,并生成了一个小的大环折叠体-肽杂合体库。最有希望的结合物随后被优化为细胞摄取并在细胞试验中进行测试。我们观察到,在该序列的n端引入一小段带正电的残基,导致抑制剂表现出独立于p53的细胞毒性活性。相反,中性乙酰化肽-折叠体大环以p53依赖的方式显示出活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
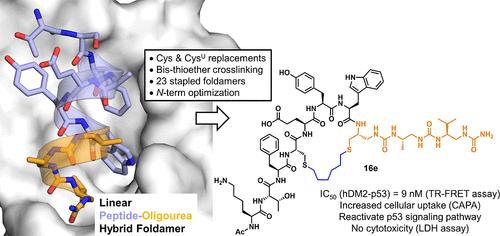
Cell-Permeable Peptide Inhibitors of the p53-hDM2 Interaction via Foldamer Helix Mimicry and Bis-Thioether Stapling
Combining helical foldamers with α-peptides can produce α-helix mimetics with a reduced peptide character and enhanced resistance to proteolysis. Previously, we engineered a hybrid peptide-oligourea sequence replicating the N-terminal α-helical domain of p53 to achieve high affinity binding to hDM2. Here, we further advance this strategy by combining the foldamer approach with side chain cross-linking to create more constrained cell-permeable inhibitors capable of effectively engaging the target within cells. Starting from the crystal structure of the foldamer-hDM2 complex, we identified specific sites suitable for stapling, and generated a small library of macrocyclic foldamer-peptide hybrids. The most promising binders were subsequently optimized for cellular uptake and tested in a cellular assay. We observed that the introduction of a short segment of positively charged residues at the N-terminus of the sequence led to inhibitors that exhibited cytotoxic activity independently of p53. In contrast, neutral acetylated peptide-foldamer macrocycles demonstrated activity in a p53-dependent manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


