菊花状NiO-CoO阳极增强锂/钠离子电池性能:对Li+/Na+电荷存储机制的洞察
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
在本研究中,采用水热法设计了一种菊花状的氧化镍-氧化钴(NiO-CoO)纳米杂化材料,作为锂离子和钠离子电池(LIB/SIB)的阳极。采用各种表征方法对合成的NiO-CoO纳米杂化物的晶体结构、化学成分和表面性能进行了分析。采用循环伏安法(CV)、恒流充放电法(GCD)和电化学阻抗谱法(EIS)研究了ni - coo纳米复合电极的锂离子和钠离子的电荷转移特性。含NiO-CoO杂化阳极的锂离子电池在0.1C-rate下的比容量为739 mA h g-1。此外,还研究了SIB的性能,在0.1 c速率下提供340 mA h g-1的比容量。有趣的是,首次组装了一个完整的电池(NiO-CoO|1M LiPF6|LiCoO2),采用NiO-CoO纳米杂化物作为锂离子电池的阳极。此外,还介绍了用于实时应用的全电池LIB,例如为商用LED灯泡和温度传感器供电。制造的全电池持续照明超过5小时,绿色LED灯泡一次充电。NiO-CoO的花状结构使得扩散路径更短,电解质接触更好,转化/再转化反应更有效,从而实现了高电荷存储。本文章由计算机程序翻译,如有差异,请以英文原文为准。
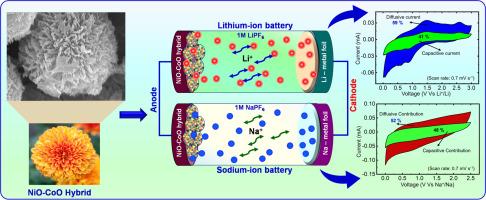
Enhanced Lithium-ion/Sodium-ion Battery Performances Using Chrysanthemum Flower-like NiO-CoO Anode: Insights into Li+/Na+ Charge Storage Mechanism
In the present work, a chrysanthemum flower-like nickel oxide-cobalt oxide (NiO-CoO) nanohybrid was designed hydrothermally to function as an anode for both lithium-ion and sodium-ion batteries (LIB/SIB). The crystal structure, chemical composition and surface properties of the synthesized NiO-CoO nanohybrid were analyzed using various characterization methods. The lithium and sodium-ion charge-transfer characteristics of the NiO-CoO nano hybrid electrode were evaluated through cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS). The LIB half-cell containing the NiO-CoO hybrid anode exhibited an excellent specific capacity of 739 mA h g-1 at 0.1C-rate. Additionally, SIB performance was also investigated, delivering 340 mA h g-1 of specific capacity at 0.1C-rate. Interestingly, a full-cell (NiO-CoO|1M LiPF6|LiCoO2) was assembled for the first time, employing the NiO-CoO nano hybrid as the anode for the LIB. Further the full cell LIB was presented for real-time applications such as powering commercial LED bulbs and a temperature sensor. The fabricated full-cell sustained illumination for over 5 hours with a green LED bulb on a single charge. The fascinating flower-like morphology of the NiO-CoO hybrid offered short diffusion path, improved electrolyte contacts and effective conversion/re-conversion reactions which in turn led to high charge storage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


