破译尿路结石的矿物密码:锌同位素的初步研究
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
锌(Zn)是所有生物必需的元素,锌同位素在研究疾病的形成中起着关键作用。尽管对健康和患病人体组织中的锌同位素进行了广泛的研究,但锌同位素在尿路结石中的作用仍未被探索。本研究利用多收集器电感耦合等离子体质谱法对37例尿路结石中的锌同位素进行了研究。尿路结石的δ66Zn值为-0.15‰~ 0.47‰,平均值为0.11‰。与草酸钙(CO)岩石(δ66Zn = -0.11‰~ 0.47‰)相比,碳酸盐磷灰石(CA)岩石具有较轻的Zn同位素组成(δ66Zn = -0.15‰~ -0.03‰)。CO和CA结石之间锌同位素组成的差异可能是由于结石形成过程中尿pH值的差异。在尿液pH值较高时,CA结石比CO结石富含较轻的Zn同位素。与血液和尿液相比,尿路结石富含较轻的锌同位素。本研究确定了肾脏运输过程中影响锌同位素变化的两个步骤。第一步是肾脏过滤和重吸收,富集尿液中的重锌同位素。第二步是尿路结石的沉积过程,其中轻同位素由于键能较低,更容易断裂。这种动力学分馏效应导致轻锌同位素在尿路结石中富集。总的来说,这项研究为影响尿路结石中锌同位素组成的地球化学机制提供了初步的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
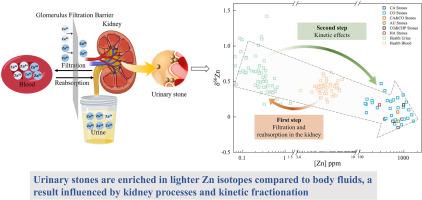
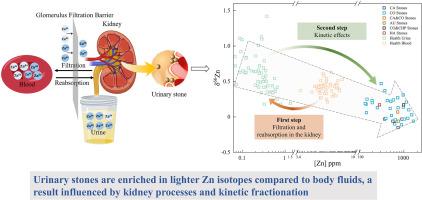
Deciphering the mineral code of urinary stones: A first look at zinc isotopes
Zinc (Zn) is an essential element for all living organisms, and Zn isotopes play a key role in studying the formation of disease. Despite extensive studies on Zn isotopes in healthy and diseased human tissues, the role of Zn isotopes in urinary stones remains unexplored. This study investigates Zn isotopes in 37 urinary stones using multi-collector inductively coupled plasma mass spectrometry. The δ66Zn values of urinary stones range from −0.15‰ to 0.47‰, with a mean value of 0.11‰. Carbonate apatite (CA) stones exhibit lighter Zn isotopic compositions (δ66Zn = −0.15‰ ∼ −0.03‰) compared to calcium oxalate (CO) stones (δ66Zn = −0.11‰ ∼ 0.47‰). The variation in Zn isotopic compositions between CO and CA stones may result from urinary pH differences during stone formation. At higher urinary pH, CA stones are enriched in lighter Zn isotopes compared to CO stones. Urinary stones are enriched in lighter Zn isotopes compared to blood and urine. This study identifies two steps influencing Zn isotope variations during kidney transport. The first step involves kidney filtration and reabsorption, enriching heavy Zn isotope in the urine. The second step is the deposition process of urinary stones, where light isotopes, due to their lower bond energy, are more prone to breaking. This kinetic fractionation effect leads to an enrichment of light Zn isotope in urinary stones. Overall, this study offers preliminary insights into the geochemical mechanisms that influence the Zn isotopic composition in urinary stones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


