Fe3+对黄铜矿表面性质和可浮性影响的实验与理论研究
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
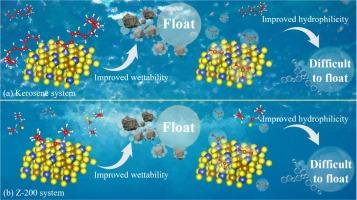
矿物加工厂的废水通常经过处理、回收和再循环。循环水中铁离子的高浓度影响了黄铜矿的浮选。因此,研究在不同捕收剂体系中引入Fe3+对黄铜矿浮选行为的影响,对实现黄铜矿的高效回收具有指导意义。结果表明,两种捕收剂均能有效促进浮选回收。当Fe3+浓度超过40 mg/L时,浮选回收率显著降低。表面性质分析表明,煤油和Z-200分别通过物理吸附和化学吸附作用于黄铜矿表面。引入Fe3+后形成的羟基铁是表面疏水性降低、电位升高的关键原因。DFT计算结果进一步表明,Z-200能与黄铜矿表面的Cu位形成稳定的Cu- s键。引入Fe3+后生成的羟基铁(Fe(OH)2+和Fe(OH)3)中的-OH和Fe分别能与矿物表面的Cu和S结合。由于羟基铁与捕收剂竞争吸附,优先吸附在黄铜矿表面,降低了浮选回收率。研究结果为降低铁离子对黄铜矿浮选的影响提供了理论支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Experimental and theoretical studies on the effect of Fe3+ on the surface properties and floatability of chalcopyrite
Wastewater from mineral processing plants is often treated, recovered, and recycled. The high concentration of iron ions in the recycled water affects the flotation of chalcopyrite. Therefore, studying the impact of introducing Fe3+ in different collector systems on the flotation behavior of chalcopyrite has guided in realizing its efficient recovery. The results show that both collectors can effectively promote flotation recovery. When the Fe3+ concentration exceeds 40 mg/L, the flotation recovery decreases significantly. Surface property analysis shows that kerosene and Z-200 act on the surface of chalcopyrite through physical adsorption and chemical adsorption, respectively. The hydroxyl iron formed after introducing Fe3+ is the crucial reason the surface hydrophobicity decreases and potential increases. DFT calculation results further demonstrate that Z-200 can form stable Cu-S bonds with Cu sites on the surface of chalcopyrite. –OH and Fe in the hydroxyl iron (Fe(OH)2+ and Fe(OH)3) generated after the introduction of Fe3+ can bond with Cu and S on the mineral surface, respectively. Because hydroxyl iron competes with the collector for adsorption, preferentially adsorbed on the surface of chalcopyrite reduced the flotation recovery. The results of this study provide theoretical support for reducing the impact of iron ions on chalcopyrite flotation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


