鱼油可降低脂多糖刺激的人前脂肪细胞中CCL2趋化因子和组蛋白修饰酶的表达。
IF 2.7
引用次数: 0
摘要
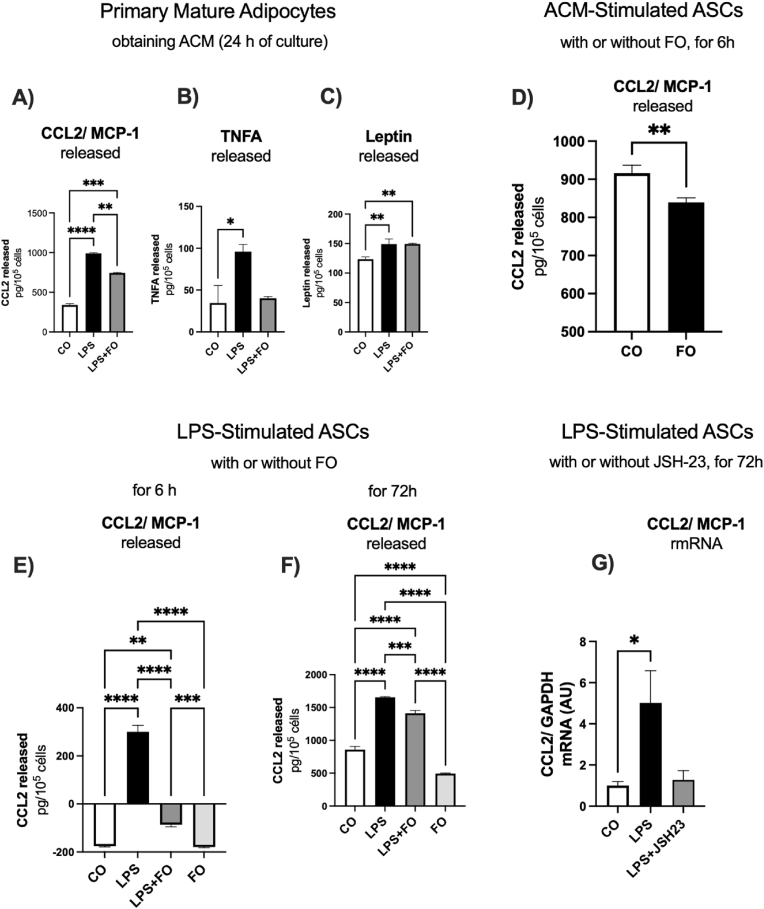
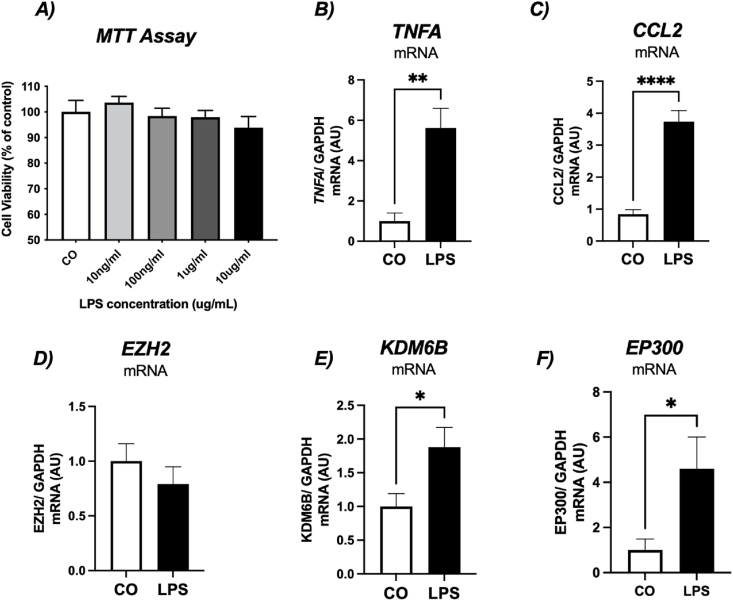
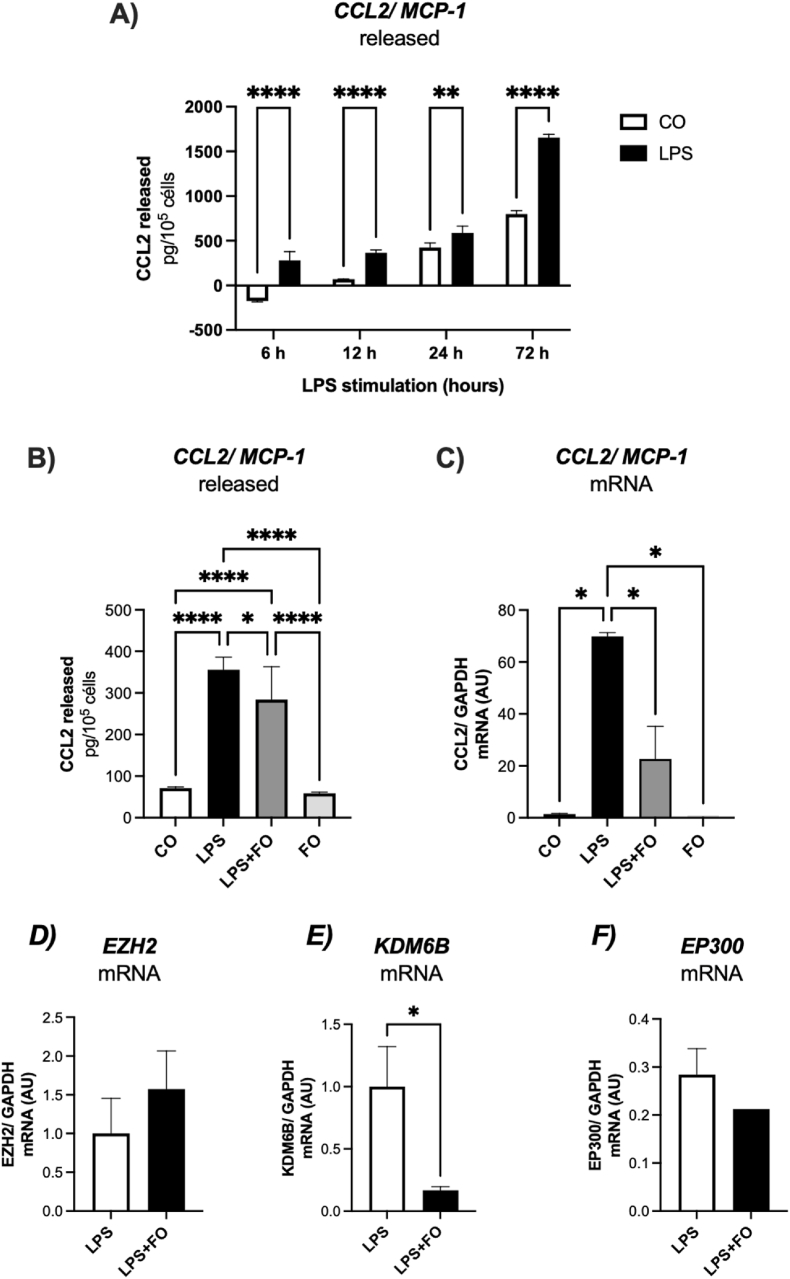
在肥胖中,C-C趋化因子配体2 (CCL2)在招募巨噬细胞到白色脂肪组织(WAT)中起关键作用,导致慢性炎症。在这项研究中,我们试图探讨鱼油(FO)对人前脂肪细胞和原代脂肪源性干细胞(ASCs)模型中CCL2表达和组蛋白(H3K27)修饰酶的影响。目前在前脂肪细胞谱系中的研究结果证明,脂多糖(LPS)增加了tnf - α(~ 5.8倍)和CCL2(~ 3.8倍)的表达,调节了H3K27修饰酶的表达,包括KDM6B和EP300。反过来,鱼油显著减弱lps诱导的CCL2表达和分泌,下调KDM6B和EP300,阐明了鱼油抗炎作用的重要作用机制。接下来,我们从超重并暴露于LPS的患者身上分离成熟的肥大脂肪细胞,导致CCL2/MCP-1(~ 3.8倍)和tnf - α(~ 4.5倍)表达增加,FO显著减弱了这种影响。我们还制备了脂肪细胞条件培养基(ACM),并将ASCs暴露于LPS或ACM中长达72小时,以评估CCL2/MCP-1的分泌情况。来自肥大脂肪细胞的ACM刺激CCL2/MCP-1表达增加,FO部分降低了CCL2/MCP-1表达。LPS处理原发性ASCs导致CCL2分泌明显增加,6 h后CCL2分泌被鱼油完全消除,显示其强大的抗炎作用。72小时后,即使在持续的炎症刺激下,FO也始终保持较低的CCL2水平,强调其调节慢性炎症的能力。此外,JSH-23对NF-κB的抑制作用与鱼油对CCL2表达的影响相似,进一步表明鱼油的抗炎作用可能与NF-κB信号传导有关。综上所述,鱼油可减弱前脂肪细胞和ASCs中CCL2的表达和分泌,证明其可能通过调节组蛋白修饰酶和炎症途径来调节WAT祖细胞的炎症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fish oil attenuates the expression of the CCL2 chemokine and histone-modifying enzymes in LPS-stimulated human preadipocytes
In obesity, C-C chemokine ligand 2 (CCL2) plays a critical role in recruiting macrophages to white adipose tissue (WAT), contributing to chronic inflammation. In this study, we sought to explore the effects of fish oil (FO) on CCL2 expression and histone (H3K27)-modifying enzymes in both human model of preadipocytes and primary adipose-derived stem cells (ASCs). Present findings in preadipocytes lineage evidenced that lipopolysaccharide (LPS) increased TNF-alpha (∼5.8-fold) and CCL2 (∼3.8-fold) expression, modulating H3K27 modifying enzymes expression, including KDM6B and EP300. FO, in turn, significantly attenuated LPS-induced CCL2 expression and secretion and downregulated KDM6B and EP300, elucidating an important mechanism of action involved in the anti-inflammatory role of FO. We next isolated mature hypertrophied adipocytes from patient with overweight and exposed to LPS, resulting in increased CCL2/MCP-1 (∼3.8-fold) and TNF-alpha (∼4.5-fold) expression, effects significantly attenuated by FO. We also generated adipocyte-conditioned medium (ACM) and exposed ASCs to LPS or ACM for up to 72 h to assess CCL2/MCP-1 secretion. ACM from hypertrophied adipocytes stimulated increased CCL2/MCP-1 expression, which was partially reduced by FO. LPS treatment of primary ASCs led to a marked increase in CCL2 secretion, which was completely abolished by FO after 6 h, highlighting its potent anti-inflammatory effect. After 72 h, FO consistently maintained lower levels of CCL2, even during sustained inflammatory stimulation, underscoring its ability to modulate chronic inflammation. Additionally, the inhibition of NF-κB with JSH-23 mimicked the effects of FO on CCL2 expression, further suggesting that the anti-inflammatory actions of FO may involve NF-κB signaling. In conclusion, FO attenuates CCL2 expression and secretion in both preadipocytes and ASCs, providing evidence of its potential in modulating inflammation in WAT progenitor cells by modulating histone-modifying enzymes and inflammatory pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism open
Agricultural and Biological Sciences (General), Endocrinology, Endocrinology, Diabetes and Metabolism
自引率
0.00%
发文量
0
审稿时长
40 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


