双set法光激发汉奇酯阴离子激活芳基乙烯的C-F键和氢二氟烷基化。
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
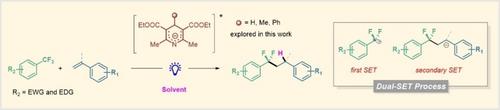
在这项研究中,我们报道了一种新的方法来激活三氟甲基芳烃的C-F键,以实现芳烯的氢二氟烷基化利用光激发汉茨酯(HEs)阴离子。合成了种类繁多的α、α-二氟烷烃。还介绍了药物分子的后期功能化和生物活性分子生物异构体的合成。机理研究表明,he阴离子的双-单电子转移(SET)过程是该反应的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photoexcited Hantzsch Ester Anions Enabled C−F Bond Activation and Hydro-Difluoroalkylation of Arylethylenes Through Dual-SET Process
In this study, we reported a new approach to activate the C−F bond of trifluoromethylarenes to achieve the hydro-difluoroalkylation of arylethylenes using photoexcited Hantzsch esters (HEs) anions. A wide range of α,α-difluoroalkanes was synthesized. Late-stage functionalization of drug molecules and synthesis of bioactive molecule bioisostere were also presented. Mechanistic studies revealed that the dual-single electron transfer (SET) process of HEs anion was the key to this reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry - A European Journal
化学-化学综合
CiteScore
7.90
自引率
4.70%
发文量
1808
审稿时长
1.8 months
期刊介绍:
Chemistry—A European Journal is a truly international journal with top quality contributions (2018 ISI Impact Factor: 5.16). It publishes a wide range of outstanding Reviews, Minireviews, Concepts, Full Papers, and Communications from all areas of chemistry and related fields.
Based in Europe Chemistry—A European Journal provides an excellent platform for increasing the visibility of European chemistry as well as for featuring the best research from authors from around the world.
All manuscripts are peer-reviewed, and electronic processing ensures accurate reproduction of text and data, plus short publication times.
The Concepts section provides nonspecialist readers with a useful conceptual guide to unfamiliar areas and experts with new angles on familiar problems.
Chemistry—A European Journal is published on behalf of ChemPubSoc Europe, a group of 16 national chemical societies from within Europe, and supported by the Asian Chemical Editorial Societies. The ChemPubSoc Europe family comprises: Angewandte Chemie, Chemistry—A European Journal, European Journal of Organic Chemistry, European Journal of Inorganic Chemistry, ChemPhysChem, ChemBioChem, ChemMedChem, ChemCatChem, ChemSusChem, ChemPlusChem, ChemElectroChem, and ChemistryOpen.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


