通过抑制析氧反应,铜掺杂NiOOH电催化将甘油转化为甲酸酯
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
电催化甘油氧化反应(GOR)与阴极析氢反应相结合,有助于降低阳极过电位,从而促进高效产氢。然而,由于高电位下析氧反应(OER)的竞争,GOR被限制在一个狭窄的电位范围内。因此,有必要开发一种在宽电位范围内具有高甲酸法拉第效率(FEFA)的催化剂。本文采用电沉积法合成了cu掺杂NiOOH催化剂,抑制了GOR过程中OER的竞争,在1.278 V vs RHE条件下获得了10 mA cm-2的电流密度,在1.3 V vs RHE ~ 1.6 V vs RHE范围内FEFA超过70.36%,在1.35 V vs RHE条件下FEFA最大达到96.46%。原位光谱研究和DFT计算表明,Cu掺杂减缓了*OH到*O的步骤以抑制OER,并增强了甘油吸附以加速GOR。提出了一种促进甘油电氧化生成甲酸酯的竞争性反应机理,为高选择性生产电催化增值化学品和可持续生产氢能提供了可行的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
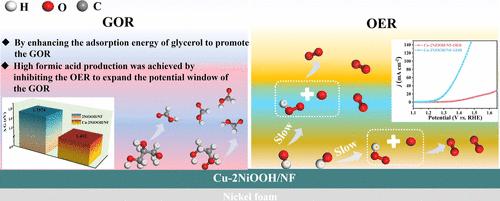
Copper-Doped NiOOH for the Electrocatalytic Conversion of Glycerol to Formate via the Inhibition of the Oxygen Evolution Reaction
The combination of the electrocatalytic glycerol oxidation reaction (GOR) with the cathodic hydrogen evolution reaction serves to reduce the anodic overpotential, thereby facilitating the efficient production of hydrogen. However, the GOR is confined to a narrow potential range due to the competition of the oxygen evolution reaction (OER) at high potential. Therefore, it is necessary to develop a catalyst with a high Faraday efficiency of formate (FEFA) over a wide potential range. Herein, Cu-doped NiOOH catalysts were synthesized by electrodeposition to inhibit the competing OER during the GOR process, achieving a current density of 10 mA cm–2 at 1.278 V vs RHE, a FEFA over 70.36% within a broad potential range of 1.3 V vs RHE to 1.6 V vs RHE, and a maximum FEFA of 96.46% at 1.35 V vs RHE. In situ spectral studies and DFT calculations revealed that Cu doping slowed the *OH to *O step for the inhibition of the OER and enhanced glycerol adsorption to accelerate the GOR. A competitive reaction mechanism for boosting glycerol electro-oxidation to formate was proposed, presenting a feasible strategy for the highly selective production of electrocatalytic value-added chemicals and the sustainable production of hydrogen energy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


