受海洋天然产物启发,发现具有抗炎活性的新型BRD4抑制剂
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
含bromodomain -containing protein 4 (BRD4)已被认为是一个很有前景的药物发现靶点,而新型特异性BRD4 bromodomain抑制剂的开发将有利于抗炎药物的发现以及bromodomain功能作用的揭示。本文以海洋喹唑啉酮类生物碱penpanoid C为灵感,设计并合成了一系列在两个芳香环体系之间具有不同连接的喹唑啉-4(3H)-。其中,化合物25具有良好的体外BRD4抑制活性(BRD4 BD1 IC50 = 3.64 μM, BRD4 BD2 IC50 = 0.12 μM)和抗炎活性(NO生成实验IC50 = 1.98 μM)。同时,25能明显抑制lps刺激的Raw 264.7和THP-1细胞中TNF-α和IL-6的表达。值得注意的是,25种在急性炎症模型中显示出体内治疗效果,没有明显的细胞毒性。这些结果表明,25是一种选择性BRD4 BD2抑制剂,是一种有前景的抗炎先导化合物,值得进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
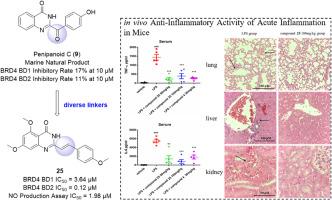
Marine natural product-inspired discovery of novel BRD4 inhibitors with anti-inflammatory activity
Bromodomain-containing protein 4 (BRD4) has been identified as a promising target in drug discovery, and the development of novel specific BRD4 bromodomain inhibitors will benefit anti-inflammatory drug discovery as well as bromodomain function role disclose. Herein, inspired by marine quinazolinone alkaloid penipanoid C, we designed and synthesized a series of quinazolin-4(3H)-ones with diverse linkers between two aromatic ring systems. Among them, compound 25 possessed good in vitro BRD4 inhibitory activities (IC50 = 3.64 μM for BRD4 BD1 and IC50 = 0.12 μM for BRD4 BD2) and anti-inflammatory activity (IC50 = 1.98 μM for NO production assay). Meantime, 25 obviously suppressed the expression of TNF-α and IL-6 in LPS-stimulated Raw 264.7 and THP-1 cells. Notablely, 25 displayed in vivo therapeutic efficacies in an acute inflammation model without obvious cytotoxicity. These findings suggest that 25 is a selective BRD4 BD2 inhibitor which is a promising anti-inflammatory lead compound worthy for further investigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


