有机磷杀虫剂马拉硫磷对斑马鱼生殖毒性和代际效应的研究(Danio rerio)。
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
以斑马鱼为模型动物,探讨了马拉硫磷(一种广泛使用的低毒有机磷杀虫剂)的生殖效应和跨代效应。采用0.1 ~ 1.0 mg/L马拉硫磷对成年斑马鱼(F0)暴露60 d,探讨性别差异的生殖毒性及其潜在机制,并对F1后代的发育和转录水平进行了评估。马拉硫磷显著地抑制了斑马鱼的生育能力,其证据是F1后代的产卵减少和受精率降低。F0斑马鱼出现性腺发育异常和类固醇激素紊乱,这与下丘脑-垂体-性腺-肝脏(HPGL)轴上核心基因(如cyp11a、cyp19a、vtg1、era)的转录改变有关。vtg1的表达水平在马拉硫磷诱导的E2和VTG的性别依赖中起关键作用。E2和VTG的降低会破坏女性卵巢功能。E2过量会导致男性女性化。分子对接表明马拉硫磷主要通过雌激素样作用和CYP11A拮抗作用诱导斑马鱼生殖障碍。父母接触马拉硫磷会使F1后代的胚胎发育异常,包括心跳减少、畸形和体长缩短。转录组学表明,马拉硫磷诱导的生殖毒性可以跨代传递,这可能对鱼类种群产生不利影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
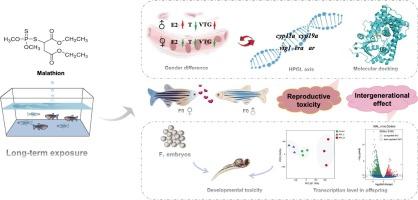
Insights into organophosphorus insecticide malathion induced reproductive toxicity and intergenerational effect in zebrafish (Danio rerio)
The reproductive and transgenerational effects of malathion, a widely utilized low-toxicity organophosphorus insecticide, were explored using zebrafish as model animal. Adult zebrafish (F0) were exposed to malathion at 0.1–1.0 mg/L for 60 days for exploring the reproductive toxicity in sex differences and the potential mechanisms, and development and transcription levels in F1 offspring were assessed. Malathion significantly suppressed the fertility of zebrafish as evidenced by reduced spawning and lower fertilization rates in F1 offspring. Abnormal gonadal development and steroid hormone disorders were observed in F0 zebrafish, which was associated with the alterations in the transcription of core genes (such as cyp11a, cyp19a, vtg1, era) along the hypothalamus-pituitary-gonad-liver (HPGL) axis. The expression level of vtg1 played a key role in the malathion-induced sex dependence on E2 and VTG levels. The reduction of E2 and VTG could disrupt ovarian capability in females. E2 excess would cause feminization in males. Molecular docking indicated that reproductive disorders induced by malathion in zebrafish mainly through estrogen-like effects and CYP11A antagonism. Parental exposure to malathion abnormalized embryonic development in F1 offspring, comprising heartbeats decrease, deformities and body length reduction. Transcriptomics suggested that malathion-induced reproductive toxicity could be transmitted across generations, which may adversely affect fish populations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


