大气二甲硫醚氧化加成通道生成甲基磺酸和SO3。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二甲基硫化物加成通道生成甲基磺酸(MSA)主要是通过甲基磺酰氧基(CH3SO3)与h原子供体的反应而非HO2自由基。在与之竞争的过程中,CH3SO3的热分解产生SO3。MSA/SO3比值受CH3SO3分解的温度依赖性影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
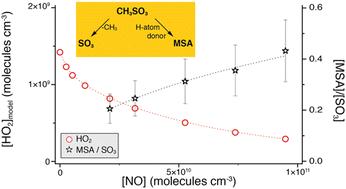
Methanesulfonic acid (MSA) and SO3 formation from the addition channel of atmospheric dimethyl sulfide oxidation†
The formation of methanesulfonic acid (MSA) from the dimethyl sulfide addition channel primarily proceeds via the reaction of methylsulfonyloxy radicals (CH3SO3) with H-atom donors, other than HO2 radicals. In competition with it, thermal decomposition of CH3SO3 results in SO3 generation. The MSA/SO3 ratio is driven by the temperature dependence of CH3SO3 decomposition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


