用于透析和纳米级分离的原子薄2D膜的蛋白质激活尺寸选择缺陷密封
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
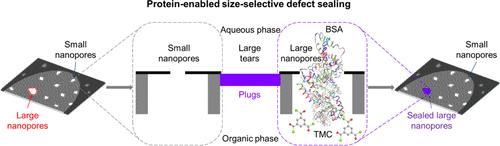
原子薄的二维材料通过高选择性(来自分子筛分)和高渗透性(由于原子薄)的结合,呈现出推进膜分离的潜力。然而,在二维材料中大面积创建高密度的精确纳米孔(窄尺寸分布)仍然具有挑战性,纳米孔非均匀性造成的非选择性泄漏会对性能产生不利影响。在这里,我们通过利用渗透蛋白的尺寸和反应性优先密封较大的纳米孔(≥4 nm),同时保留大量较小的纳米孔(通过位阻),证明了在厘米尺度上原子薄石墨烯膜的蛋白质激活尺寸选择性缺陷密封(PDS)。我们的缺陷密封纳米多孔原子薄膜(natm)在与模型透析生物分子异硫氰酸荧光素(FITC)-Ficoll 70在磷酸盐缓冲盐水(PBS)溶液中进行尺寸选择性扩散分离时显示出长达35天的稳定性,并且优于最先进的市售透析膜(分子量截止~ 3.5-5 kDa和~ 8-10 kDa),对较小溶质KCl (~ 0.66 nm) ~ 5.1-6 × 10-5 ms-1和维生素B12 (B12,与小蛋白溶菌酶(Lz, ~ 4 nm) ~ 4 - 6.4 × 10-8 m s-1相比,对B12/Lz ~ 70和KCl/Lz ~ 1280具有前所未有的选择性。我们的工作将蛋白质作为纳米级工具,用于在原子薄膜上进行尺寸选择缺陷密封,以克服持续存在的问题,并推进透析、蛋白质脱盐、小分子分离/纯化和其他生物过程的分离。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Protein-Enabled Size-Selective Defect-Sealing of Atomically Thin 2D Membranes for Dialysis and Nanoscale Separations
Atomically thin 2D materials present the potential for advancing membrane separations via a combination of high selectivity (from molecular sieving) and high permeance (due to atomic thinness). However, the creation of a high density of precise nanopores (narrow-size-distribution) over large areas in 2D materials remains challenging, and nonselective leakage from nanopore heterogeneity adversely impacts performance. Here, we demonstrate protein-enabled size-selective defect sealing (PDS) for atomically thin graphene membranes over centimeter scale areas by leveraging the size and reactivity of permeating proteins to preferentially seal larger nanopores (≥4 nm) while preserving a significant amount of smaller nanopores (via steric hindrance). Our defect-sealed nanoporous atomically thin membranes (NATMs) show stability up to ∼35 days during size-selective diffusive separations with a model dialysis biomolecule fluorescein isothiocyanate (FITC)-Ficoll 70 in phosphate buffer saline (PBS) solution as well as outperform state-of-the-art commercially available dialysis membranes (molecular-weight-cutoff ∼3.5–5 kDa and ∼8–10 kDa) with significantly higher permeance for smaller solutes KCl (∼0.66 nm) ∼5.1–6 × 10–5 ms–1 and vitamin B12 (B12, ∼1.5 nm) ∼2.8–4 × 10–6 ms–1 compared to small protein lysozyme (Lz, ∼4 nm) ∼4–6.4 × 10–8 m s–1, thereby allowing unprecedented selectivity for B12/Lz ∼70 and KCl/Lz ∼1280. Our work introduces proteins as nanoscale tools for size-selective defect sealing in atomically thin membranes to overcome persistent issues and advance separations for dialysis, protein desalting, small molecule separations/purification, and other bioprocesses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


