ph可调脂质构象变化的分子动力学模拟
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
ph敏感脂质是脂质纳米颗粒的重要组成部分,它可以实现药物的靶向递送和控制释放。在分子水平上了解ph触发药物释放的机制对于合理设计可电离脂质具有重要意义。基于最近报道的一种名为SL2的ph可切换脂质,采用分子动力学(MD)模拟方法探讨了ph可切换脂质构象变化引起膜不稳定的微观机制。模拟结果表明,在中性pH下,中性SL2脂质与其他组分(辅助脂质和胆固醇)组装形成结构有序的双层结构。此时,SL2的两条烃链紧密排列并有序插入膜内。随着pH的降低,吡啶环的质子化引起了很大程度的分子结构变化。吡啶环倾向于与甲氧基形成分子内氢键,与水形成分子间氢键,导致吡啶环翻转。同时,由于结构翻转,两个烷烃链呈现出更开放的状态,从而扰乱了膜内分子的排列。这种扰动引起膜的局部坍塌和水分子通道的形成,从而导致ph诱导的药物释放。我们的结果在分子水平上验证了实验提出的机制,并提供了更多的补充信息,有望对ph触发的药物释放有更深入的了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
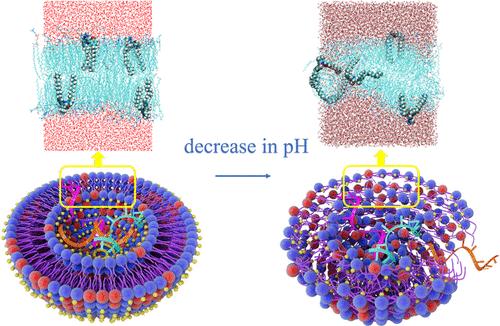
Molecular Dynamics Simulation on the Conformational Change of a pH-Switchable Lipid
pH-sensitive lipids are important components of lipid nanoparticles, which enable the targeted delivery and controlled release of drugs. Understanding the mechanism of pH-triggered drug release at the molecular level is important for the rational design of ionizable lipids. Based on a recently reported pH-switchable lipid, named SL2, molecular dynamics (MD) simulations were employed to explore the microscopic mechanism behind the membrane destabilization induced by the conformational change of pH-switchable lipids. The simulated results showed that, at neutral pH, the neutral SL2 lipids assembled with other components (helper lipids and cholesterol) to form a structurally ordered bilayer structure. At this moment, the two hydrocarbon chains of SL2 were closely aligned and inserted in an orderly manner inside of the membrane. With a decrease in pH, the protonation of the pyridinium ring caused a large degree of molecular structural change. The pyridinium ring preferred to form intramolecular H-bonds with the methoxy groups and intermolecular H-bonds with water, resulting in the flip of the pyridinium ring. Meanwhile, due to the structural flip, the two alkane chains showed a more open state, which perturbed the arrangement of molecules within the membrane. The perturbations caused local collapse of the membrane and the formation of water molecule channels, which contributed to the pH-induced drug release. Our results verified the experimentally proposed mechanism at the molecular level and provided more complementary information, which are expected to have deeper insights into the pH-triggered drug release.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


