低水化条件下吸水对聚醚电解质离子电导率的影响
IF 5.2
Q1 POLYMER SCIENCE
引用次数: 0
摘要
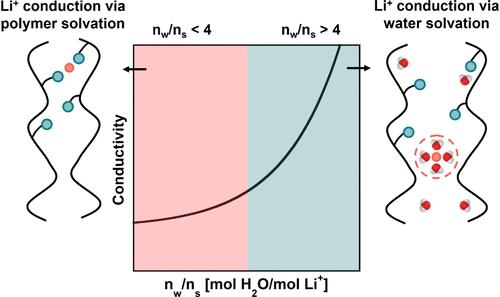
含离子聚合物在电化学和水处理应用中受到广泛的水化条件的影响。用于电池的干聚合物电解质和用于水净化的高度膨胀膜的重大工作使我们了解了极端条件下离子传输的情况。然而,对中间条件(即低水合作用)的了解对于新兴应用(如电解槽、燃料电池和锂提取)至关重要。低水化水平下的离子传输不同于通常研究的极端条件,相关的物理不能从现有的知识中推断出来,这阻碍了材料的设计。在这项研究中,我们对在干燥条件下系统水化的litfsi掺杂聚醚进行了离子传输测量。研制了一种在湿度控制下对薄膜聚合物的吸水率和离子电导率进行平行测量的半自动化装置。对于本研究考虑的材料和膨胀范围(即0.07 g水/g干聚合物电解质),离子电导率与吸水率呈非线性关系,初始吸水率对电导率影响较弱。随着溶胀的增加,观察到电导率的显著增加。值得注意的是,水吸附引起的电导率变化与每个锂离子的水分子数相关,不同样品的归一化摩尔电导率有效地相互坍塌,直到该水化单位超过锂离子在水条件下的溶剂化数。这些结果提供了关于痕量水污染对聚合物电解质电导率测量影响的重要知识,并表明锂离子溶剂化数标志着水对含离子聚合物中离子传输影响的关键转折点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Influence of Water Sorption on Ionic Conductivity in Polyether Electrolytes at Low Hydration
Ion-containing polymers are subject to a wide range of hydration conditions across electrochemical and water treatment applications. Significant work on dry polymer electrolytes for batteries and highly swollen membranes for water purification has informed our understanding of ion transport under extreme conditions. However, knowledge of intermediate conditions (i.e., low hydration) is essential to emerging applications (e.g., electrolyzers, fuel cells, and lithium extraction). Ion transport under low levels of hydration is distinct from the extreme conditions typically investigated, and the relevant physics cannot be extrapolated from existing knowledge, stifling materials design. In this study, we conducted ion transport measurements in LiTFSI-doped polyethers that were systematically hydrated from dry conditions. A semiautomated apparatus that performs parallel measurements of water uptake and ionic conductivity in thin-film polymers under controlled humidity was developed. For the materials and swelling range considered in this study (i.e., <0.07 g water/g dry polymer electrolyte), ionic conductivity depends nonlinearly on water uptake, with the initial sorbed water weakly affecting conductivity. With additional increases in swelling, more significant increases in conductivity were observed. Remarkably, changes in conductivity induced by water sorption were correlated with the number of water molecules per lithium ion, with the normalized molar conductivity of different samples effectively collapsing onto one another until this unit of hydration exceeded the solvation number of lithium ions under aqueous conditions. These results provide important knowledge regarding the effects of trace water contamination on conductivity measurements in polymer electrolytes and demonstrate that the lithium-ion solvation number marks a key transition point regarding the influence of water on ion transport in ion-containing polymers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


