二甲联苯- salen钇配合物诱导β-内酯的共二选择开环聚合
IF 5.2
Q1 POLYMER SCIENCE
引用次数: 0
摘要
聚羟基烷酸酯(PHAs)已成为传统石油基塑料的有前途的替代品。通过外消旋β-内酯的立体控制开环聚合(ROP)化学合成立体规则相芳烃是一种具有巨大挑战的理想策略。在此,我们开发了一类DiMeBiPh-salen钇配合物,它们采用顺式-α构型用于rac-β-丁内酯(rac- bbl)和rac-β-戊内酯(rac- bvl)的立体选择性ROP。值得注意的是,催化剂Y5促进了稳定的聚合,TOF高达104 h-1,并提供了共规P3HB, P3HV和P(3HB)-co-P(3HV)共聚物,Pr值高达0.95。改变P(3HB)-co-P(3HV)共聚物的组成为微调热性能提供了一个有趣的机会。我们的动力学研究支持聚合物交换机制。这项工作表明,DiMeBiPh-salen体系可以作为β-内酯立体选择性ROP的新催化框架,利用了立体选择性聚合的催化剂设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
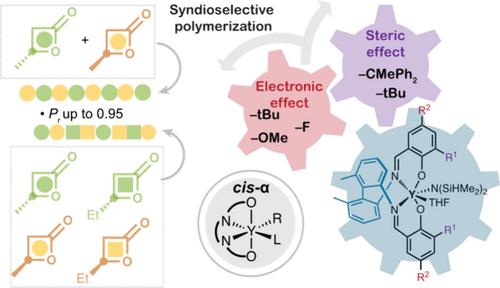
Syndioselective Ring-Opening Polymerization of β-Lactones Enabled by Dimethylbiphenyl-Salen Yttrium Complexes
Polyhydroxyalkanoates (PHAs) have served as promising alternatives to traditional petroleum-based plastics. Chemical synthesis of stereoregular PHAs via stereocontrolled ring-opening polymerization (ROP) of racemic β-lactones was a desired strategy with a formidable challenge. Herein, we developed a class of DiMeBiPh-salen yttrium complexes that adopted a cis-α configuration for stereoselective ROP of rac-β-butyrolactones (rac-BBL) and rac-β-valerolactone (rac-BVL). Notably, catalyst Y5 promoted robust polymerization with TOF up to 104 h–1 and furnished syndiotactic P3HB, P3HV, and P(3HB)-co-P(3HV) copolymers with Pr values of up to 0.95. Varying the compositions in P(3HB)-co-P(3HV) copolymers offered an intriguing opportunity to fine tune the thermal properties. Our kinetic study supported a polymeryl exchange mechanism. This work demonstrated that the DiMeBiPh-salen system could serve as a new catalytic framework for the stereoselective ROP of β-lactones, which leverages the catalyst design for stereoselective polymerization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


