用铜金属辅助化学蚀刻硅:揭示Cu2O在微尺度结构制造中的作用
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
在硅三维(3D)结构制造领域,实现精确和经济的蚀刻仍然是一个重大挑战。本文采用Cu-metal-assisted chemical etching (Cu-MACE)技术成功制备了各向异性为0.73的微尺度Si结构,并提出了Cu在MACE溶液中的化学行为机制。我们的研究揭示了过氧化氢(H2O2)存在下Cu薄膜内氧化亚铜(Cu2O)的形成,这在Si蚀刻中起着关键作用。我们提出Cu2O还原为Cu时产生的空穴被转移到Si上,通过与Cu2O的电反应促进其蚀刻。在合适的条件下,在Si - cu2o原电池中进行Cu-Cu2O循环氧化还原过程,实现了Si的连续蚀刻,显著提高了Cu-MACE的化学稳定性。基于这种循环过程机制,我们通过直接使用薄膜和颗粒形式的Cu2O,而不是从Cu开始,证明了Cu2O对氧化物辅助化学蚀刻(OACE)的催化潜力。本研究为Cu-MACE的精确控制提供了可能,扩展了现有的MACE机制,并有助于我们对过渡金属氧化物在OACE中的行为的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
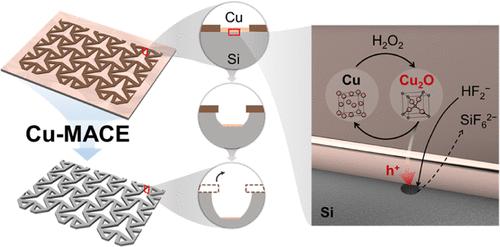
Silicon Etching Using Copper–Metal-Assisted Chemical Etching: Unveiling the Role of Cu2O in Microscale Structure Fabrication
Achieving precise and cost-effective etching in the field of silicon three-dimensional (3D) structure fabrication remains a significant challenge. Here, we present the successful fabrication of microscale anisotropic Si structures with an etching anisotropy of 0.73 using Cu-metal-assisted chemical etching (Cu-MACE) and propose a mechanism to elucidate the chemical behavior of Cu within the MACE solution. Our study reveals the formation of cuprous oxide (Cu2O) within Cu thin films in the presence of hydrogen peroxide (H2O2), which plays a key role in Si etching. We propose that the holes generated through the reduction of Cu2O back to Cu are transferred to Si, promoting its etching through a galvanic reaction with Cu2O. This Cu–Cu2O cyclic redox process in the Si–Cu2O galvanic cell under the right conditions enables continuous etching of Si and significantly improves the chemical stability of Cu-MACE. Building on this cyclic process mechanism, we demonstrate the catalytic potential of Cu2O for oxide-assisted chemical etching (OACE) by directly using Cu2O in both thin-film and particle forms, rather than starting from Cu. This study opens possibilities for the precise control of Cu-MACE, extends the existing MACE mechanism, and contributes to our understanding of transition metal oxide behavior in OACE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


