喹啉在醇与惰性甲基C(sp3) -氢键脱氧偶联反应中的分子内氢化物穿梭
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-12-09
DOI:10.1021/acs.joc.4c0226910.1021/acs.joc.4c02269
引用次数: 0
摘要
本文报告的是通过氢化物转移引发的脱氧偶联反应,在未活化的 C(sp3)-H 键上形成 C(sp3)-C(sp3)键。在无金属条件下提供了各种多环氢喹啉,并具有极好的非对映选择性。机理研究表明,喹啉作为分子内氢化物穿梭器,依次实现了氢化物的抽取和释放。该方法不仅为 C(sp3)-C(sp3)键形成的直接脱氧偶联提供了一种实用的策略,而且还开发了一种涉及喹啉促成的氢化物转移的新反应类型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
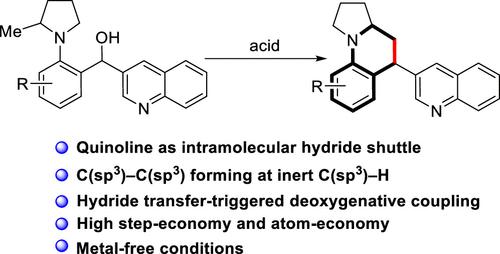
Quinoline as an Intramolecular Hydride Shuttle in the Deoxygenative Coupling Reaction of Alcohol and the Inert Methyl C(sp3)–H Bond
Reported herein is the C(sp3)–C(sp3) bond-forming at an unactivated C(sp3)–H bond via hydride transfer-initiated deoxygenative coupling reactions. Various polycyclic hydroquinolines were provided under metal-free conditions with excellent diastereoselectivity. Mechanistic study revealed that quinoline served as an intramolecular hydride shuttle to achieve the hydride abstraction and release in order. This methodology not only provides a practical strategy for direct deoxygenative coupling for the C(sp3)–C(sp3) bond-forming but also develops a new reaction type involving quinoline-enabled hydride transfer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


