大块和表面Li6PS5Cl银柱石的机械和电子特性:对锂丝电阻的第一性原理见解
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
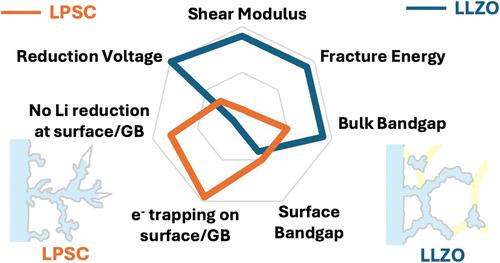
在石榴石Li7La3Zr2O12 (LLZO)和银汞石Li6PS5Cl (LPSC)等具有高离子电导率的固体电解质(SEs)中,实验观察到不同的锂丝生长模式。在此,我们利用密度泛函理论计算方法探讨了LPSC的力学和电子特性,并与其他se进行了比较,以确定预测Li-filament resistance的相关描述符。LPSC结构复杂,可以进行S2 - /Cl -倒转,Li+分布在两个Wyckoff位点(24g和48h)。通过系统的结构取样,确定了一种具有代表性的结合了这两种现象的体结构。在松弛48h后,能量最低的块体结构中Li+占绝大多数,与实验研究结果一致。块状LPSC的杨氏模量和剪切模量较低,约为10-30 GPa,沿(100)- li2s缺陷表面切割的断裂能也较低,为0.20 J/m2,表明其对长丝生长的机械阻力较差。LPSC的裂纹表面和孔隙表面具有与体块LPSC相似的带隙和多余电子分布,表明这些内部缺陷不会捕获电子将Li+还原为Li-metal。因此,LPSC很可能经历“干”裂缝,首先是机械裂缝打开,然后是锂丝填充裂缝。这与LLZO相反,LLZO具有较高的断裂能,并且在内部缺陷(例如裂纹表面、孔隙表面和晶界)经历电子局域化。LLZO已被实验观察到遭受“湿”裂纹。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanical and Electronic Properties of Bulk and Surface Li6PS5Cl Argyrodite: First-Principles Insights on Li-Filament Resistance
Different Li-filament growth patterns have been experimentally observed in numerous solid electrolytes (SEs) with high ionic conductivity such as garnet Li7La3Zr2O12 (LLZO) and argyrodite Li6PS5Cl (LPSC). Herein, we probed the mechanical and electronic properties of LPSC, using density functional theory calculations, and compared with other SEs to determine the relevant descriptors for predicting Li-filament resistance. LPSC has a complicated structure that can incorporate S2–/Cl– inversion and has Li+ distributed among two Wyckoff sites (24g and 48h). A representative bulk structure that incorporates both phenomena was determined via systematic structure sampling. The lowest energy bulk structures had a majority of Li+ in 48h sites after relaxation, agreeing with experimental studies. The Young’s modulus and shear modulus of bulk LPSC are low, ∼10–30 GPa, and the fracture energy of cleaving along the (100)-Li2S-deficient surface is also low, 0.20 J/m2, suggesting poor mechanical resistance to filament growth. The crack surfaces and pore surfaces in LPSC have a similar bandgap and excess electron distribution compared to bulk LPSC, suggesting that these internal defects will not trap electrons to reduce Li+ to Li-metal. Thus, LPSC is likely to experience “dry” cracks, with a mechanical crack opening up first, followed by a Li-filament filling the crack. This is opposite to LLZO, which has a high fracture energy and experiences electron localization at internal defects (e.g., crack surfaces, pore surfaces, and grain boundaries). LLZO has been experimentally observed to suffer “wet” cracks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


