铜绿假单胞菌脂加氧酶合成8S-和11s -羟基二碳四烯酸的底物结合腔工程
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
脂加氧酶催化多不饱和脂肪酸的双氧作用。值得注意的是,大多数微生物脂氧合酶,包括铜绿假单胞菌的脂氧合酶(Pa-LOX),分别催化亚油酸和花生四烯酸氧化成13s -氢过氧十八烯酸(13S-HPODE)和15s -氢过氧二十碳四烯酸(15S-HPETE)。因此,本研究的重点是对Pa-LOX的位置特异性或区域选择性进行修饰。亚油酸氧合和底物对接模拟表明,Pa-LOX的区域选择性可能取决于烃尾结合腔的几何形状。因此,扩大底物结合腔的内端,使C10而不是C13面对铁活性位点。值得注意的是,M434G突变导致花生四烯酸的主要氧合产物从15s -羟基二十碳四烯酸(15S-HPETE)变为11S-HPETE。另一方面,Y609G的取代使得花生四烯酸形成8S-HPETE。8S-HPETE经大肠杆菌全细胞生物催化、溶剂萃取、硅胶层析、盐酸三(2-羧基乙基)膦还原,分离得率为62%,纯度为94%。这是花生四烯酸在高转化率下生产11S-HPETE和8S-HPETE的首次报道。因此,本研究为可再生脂肪酸可持续制备具有生物活性的氧化脂类提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
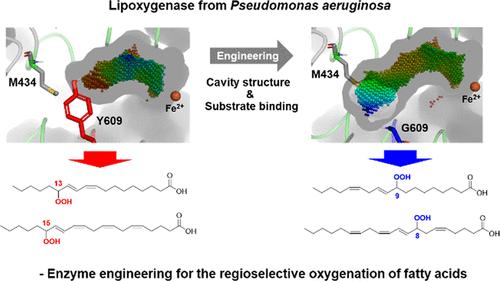
Substrate-Binding Cavity Engineering of the Lipoxygenase from Pseudomonas aeruginosa to Produce 8S- and 11S-Hydroxyeicosatetraenoic Acids
Lipoxygenases catalyze the dioxygenation of polyunsaturated fatty acids. Notably, most microbial lipoxygenases including the lipoxygenase from Pseudomonas aeruginosa (Pa-LOX) catalyze oxygenation of linoleic acid and arachidonic acid into 13S-hydroperoxyoctadecenoic acid (13S-HPODE) and 15S-hydroperoxyeicosatetraenoic acid (15S-HPETE), respectively. Therefore, this study has focused on modification of positional specificity or regioselectivity of Pa-LOX. The linoleic acid oxygenations and substrate-docking simulations suggested that the regioselectivity of Pa-LOX might depend on the geometry of the hydrocarbon tail-binding cavity. Therefore, the interior end of the substrate-binding cavity was enlarged to make C10 instead of C13 face the iron active site. Remarkably, the M434G mutation led to alteration of the oxygenation products from 15S-hydroxyeicosatetraenoic acid (15S-HPETE) to 11S-HPETE as the major product from arachidonic acid. On the other hand, the Y609G substitution allowed the formation of 8S-HPETE from arachidonic acid. 8S-HPETE was recovered after reduction by tris(2-carboxyethyl)phosphine hydrochloride with an isolated yield of 62% with a purity of 94% via Escherichia coli-based whole-cell biocatalysis, solvent extraction, and silica gel chromatography. This is the first report of the production of 11S-HPETE and 8S-HPETE from arachidonic acid at high conversions. Therefore, this study contributes to the preparation of biologically active oxylipins from renewable fatty acids in a sustainable way.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


