肠道菌群缺失通过调节小鼠铁下垂减轻铁超载诱导的结肠炎
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
铁超载破坏肠道菌群,诱发铁下垂,导致结肠炎。然而,肠道菌群是否直接驱动铁超载性结肠炎及其潜在机制尚不清楚。目的探讨肠道菌群是否可直接调控铁超载性结肠炎及其机制。方法采用硫酸亚铁喂养C57BL/6N小鼠,建立铁超载模型。使用抗生素和DSS分别建立无菌结肠炎模型和结肠炎模型。结果结果表明,铁超载通过激活促炎症反应,破坏全身铁稳态,诱导铁下沉,最终导致小鼠结肠炎。值得注意的是,铁超载抑制了System Xc-并激活了核因子e2相关因子2/血红素氧合酶-1通路,驱动铁上塌和结肠炎的进展。用高剂量柠檬酸铁铵处理小鼠结肠上皮细胞也观察到类似的结果。此外,铁超载通过激活铁下垂和增加有害细菌(如Mucispirillum)丰度加重了dss诱导的结肠炎。有趣的是,消除肠道微生物群可以减轻铁超载引起的结肠炎,而不会通过抑制小鼠铁下垂而影响全身炎症。肠道菌群的消耗通过抑制小鼠铁下垂部分减轻了铁超载对dss诱导的结肠炎的加重作用。结论铁超载激活小鼠结肠细胞铁凋亡,增加有害细菌的相对丰度,加重dss诱导的小鼠结肠炎。铁超载以微生物依赖的方式加剧了dss诱导的铁下垂和结肠炎。针对肠道微生物群可能为管理铁超载引起的结肠炎提供新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
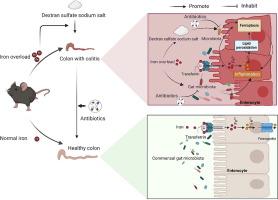

Absence of gut microbiota alleviates iron overload-induced colitis by modulating ferroptosis in mice
Introduction
Iron overload disrupts gut microbiota and induces ferroptosis, contributing to colitis. However, whether gut microbiota directly drives iron overload-induced colitis and its underlying mechanism remain unclear.
Objectives
The study aimed to explore whether gut microbiota can directly regulate iron overload-induced colitis and its underling mechanism.
Methods
Male C57BL/6N mice were fed with ferrous sulfate to establish an iron overload model. Antibiotics and dextran sulfate sodium salt (DSS) were used to create germ-free and colitis models, respectively.
Results
Results showed that iron overload caused disruption of systemic iron homeostasis via activating pro-inflammation response, which caused induction of ferroptosis and eventually resulted in colitis in mice. Notably, iron overload inhibited System Xc- and activated the nuclear factor E2-related factor 2/heme oxygenase-1 pathway, driving ferroptosis and colitis progression. Similar results were observed in mouse colon epithelial cells, which were treated with high doses ferric ammonium citrate. Additionally, iron overload exacerbated DSS-induced colitis by activating the ferroptosis and increasing harmful bacteria (e.g., Mucispirillum) abundance. Interestingly, eliminating gut microbiota attenuated iron overload-induced colitis, without affecting systemic inflammation through inhibiting ferroptosis of mice. Depletion of the gut microbiota partially mitigated the exacerbating effect of iron overload on DSS-induced colitis through inhibiting ferroptosis of mice.
Conclusion
Iron overload activates ferroptosis in colonic cells, increases the relative abundance of harmful bacteria, and exacerbates DSS-induced colitis in mice. Iron overload exacerbates DSS-induced ferroptosis and colitis in a microbiota-dependent manner. Targeting gut microbiota may offer new strategies for managing iron overload-induced colitis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


