铜和铈在Cu/CeZSM催化剂中甲烷直接制甲醇反应的作用:构效关系的认识
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
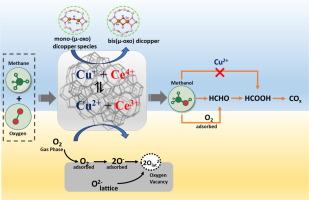
采用湿浸渍法制备了几种CeO2-ZSM5、CeO2-SiO2和CeO2-Al2O3负载CuO催化剂,并采用N2吸附解吸、x射线衍射(XRD)、傅里叶变换红外光谱(FTIR)、x射线光电子能谱(XPS)等技术对其进行了表征,研究了不同反应参数(温度、质量、小时空速(WHSV)和CH4:O2)下,甲烷直接转化为甲醇(DMTM)。紫外可见光谱(UV-vis)、程序升温还原(H2-TPR)、程序升温解吸(CO2-TPD)和co漫反射红外傅立叶变换光谱(CO-DRIFT)的研究。表征结果证实了双(µ-oxo)二铜或单(µ-oxo)二铜的形成被认为是DMTM反应的活性位点。此外,不同的铜(Cu2+, Cu+和Cu0)和铈(Ce3+和Ce4+)在氧化还原循环平衡(Cu+ + Ce4+ + + + Cu2+ + Ce3+)中共存,这取决于CeO2的负载。XPS研究表明,CeO2沉积过程中晶格氧和吸附氧的生成,其比例随CeO2负载的变化而变化。此外,不同种类的铈在催化剂表面引起电荷不平衡、氧空位和不饱和化学键的形成。CeO2和CuO的沉积在xCu/yCe-ZSM5复合催化剂中结合了更多的刘易斯酸位点,甚至形成了来自交换铜的额外的刘易斯酸位点。复合催化剂中形成的多种铜和铈对甲烷分子的活化、甲醇的选择性和产率有很大影响。表面Cu2+促进甲醇的形成,防止甲醇过度氧化生成氧化物和二氧化碳。除Cu2+外,CeO2沉积过程中产生的晶格和吸附氧也影响甲醇的形成和氧化。因此,最佳的Cu2+表面浓度和吸附氧的晶格可使甲醇收率最大化。工艺参数对甲烷转化率、甲醇选择性和产率也有影响。采用20Cu/15CeZ催化剂,在873 K, 1030 ml hr-1 gcat -1和CH4:O2 = 2:1条件下,甲醇选择性为6.34 %,甲烷转化率为37.89 %。基于甲醇氧化活性和原位DRFIT研究,提出了甲烷氧化制甲醇的合理反应机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Role of copper and cerium species in Cu/CeZSM catalysts for direct methane to methanol reaction: Insights of structure–activity relationship
The direct methane to methanol (DMTM) conversion was studied in a fixed bed reactor under varying reaction parameters including temperature, weight hourly space velocity (WHSV) and CH4:O2 over several CeO2-ZSM5, CeO2-SiO2 and CeO2-Al2O3 supported CuO catalysts prepared by wetness impregnation method and characterized by several techniques including N2 adsorption desorption, X-ray diffraction (XRD), Fourier transformed infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), ultraviolet visible spectroscopy (UV–vis), temperature programmed reduction (H2-TPR), temperature programmed desorption (CO2-TPD) and CO-diffuse reflectance infrared Fourier transform spectroscopy (CO-DRIFT) studies. The characterization results confirm the formation of bis(µ-oxo) dicopper or mono-(µ-oxo) dicopper species considered as the active sites for the DMTM reaction. Moreover, various copper (Cu2+, Cu+ and Cu0) and cerium (Ce3+ and Ce4+) species are coexisted in a redox cycle equilibrium (Cu+ + Ce4+ ⇌ Cu2+ + Ce3+) depending on CeO2 loading. XPS studies indicates the generation of lattice and adsorbed oxygen species on deposition of CeO2 and their ratio varied with the CeO2 loading. Moreover, the different cerium species induces charge unbalance, oxygen vacancies, and formation of unsaturated chemical bonds on the catalyst’s surface. The deposition of CeO2 and CuO incorporates more Lewis’s acid sites in xCu/yCe-ZSM5 composite catalysts and even formation of additional Lewis’s acid sites originated from exchanged copper species. The various copper and cerium species formed in the composite catalysts strongly influenced the methane molecule activation, and selectivity and yield of methanol. The surface Cu2+ species promotes the formation of methanol and prevents the methanol overoxidation forming oxygenates and carbon dioxide. In addition to the Cu2+ species, the lattice and adsorbed oxygen generated on deposition of CeO2 also influence the formation and oxidation of methanol. Thus, optimum surface concentration of Cu2+ and lattice to adsorbed oxygen maximizes the yield of methanol. The process parameters also the affect the methane conversion and methanol selectivity and yield. The methanol selectivity of 6.34 % with methane conversion of 37.89 % was achieved over 20Cu/15CeZ catalysts at 873 K, 1030 ml hr-1 gcat−1 and CH4:O2 = 2:1. A plausible reaction mechanism of oxidation of methane to methanol based on the activity results and in-situ DRFIT studies of methanol oxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


