西他沙星关键成分顺-2-氟环丙基羧酸的高选择性不对称合成策略研究
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-27
DOI:10.1021/acs.joc.4c0229910.1021/acs.joc.4c02299
引用次数: 0
摘要
以市售的氟甲基苯基砜和手性甘油基衍生物为原料,以100 g为起始原料,开发了具有良好立体选择性和区域选择性的顺式-2-氟环丙烷羧酸的高精度不对称合成路线。尽管总产率很高,但合成路线因其简洁而引人注目。这一战略为进一步以工业规模生产西他沙星奠定了基础,并提供了负担得起的高质量产品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
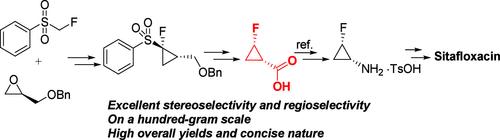
Investigation of a Highly Selective Asymmetric Synthesis Strategy for cis-2-Fluorocyclopropanecarboxylic Acid: The Key Component of Sitafloxacin
A high-precision asymmetric synthetic route for cis-2-fluorocyclopropanecarboxylic acid with excellent stereoselectivity and regioselectivity was developed from commercially available starting materials, namely, fluoromethylphenylsulfone and chiral glycidyl derivatives, on a 100 g scale at the start. Despite the high overall yield, the synthetic route is remarkable for its brevity. This strategy forms the basis for further production of sitafloxacin on an industrial scale and provides an affordable and high-quality product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


