IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
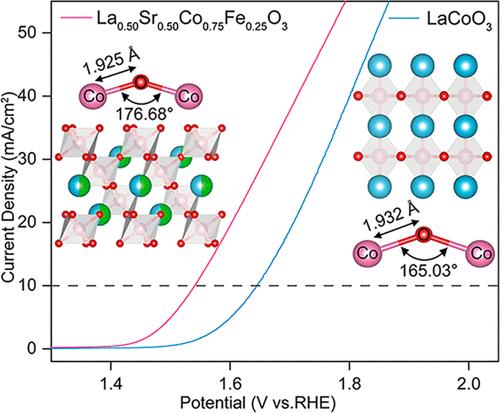
过氧化物催化剂中金属和氧之间的轨道杂化可以降低过电位,提高氧进化反应(OER)活性。本研究将密度泛函理论与实验相结合,阐明了 Sr/Fe 共掺如何调节 LaCoO3 的轨道杂化并提高其 OER 催化活性。制备的 La0.50Sr0.50Co0.75Fe0.25O3 表现出卓越的性能,在 10 mA cm-2 电流密度下过电位低至 310 mV,塔菲尔斜率为 107.03 mV dec-1,优于大多数最先进的基于包晶石的 OER 电催化剂。实验结果证实,Sr/Fe 共掺可增强 LaCoO3 中 Co-O-Co 键角度的扩展,加强 Co-O 键的共价性,从而提高电催化活性。此外,增加 Sr 掺杂会减小 Co 3d/O 2p 中心与费米级之间的距离,从而减小它们之间的能差、提高 Co 3d 与 O 2p 之间的轨道杂化程度。随着 Co 3d/O 2p 轨道杂化程度的增加,活性中心与中间产物 OOH 之间的电荷转移也随之增加,从而降低了决定速率步骤的能垒,同时降低了过电位。这项研究为基于轨道杂化的 OER 催化剂的合理设计提供了深入的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Controlling Co 3d/O 2p Orbital Hybridization in LaCoO3 by Modulating the Co–O–Co Bond Angle for Enhanced Oxygen Evolution Reaction Catalysis
The orbital hybridization between metal and oxygen of perovskite catalysts can lower the overpotential and enhance the oxygen evolution reaction (OER) activity. This study combines density functional theory with experiments to clarify how Sr/Fe codoping modulates orbital hybridization and enhances OER catalytic activity of LaCoO3. The as-prepared La0.50Sr0.50Co0.75Fe0.25O3 shows remarkable performance with a low overpotential of 310 mV at 10 mA cm–2 current density and a 107.03 mV dec–1 Tafel slope, outperforming most state-of-the-art perovskite-based OER electrocatalysts. The experimental results confirm that Sr/Fe codoping enhances the expansion of Co–O–Co bond angles and strengthens the covalency of the Co–O bond in LaCoO3, leading to enhanced electrocatalytic activity. Moreover, increasing Sr doping reduces the distance between the Co 3d/O 2p center and the Fermi level, decreasing the energy difference between them and enhancing the degree of orbital hybridization between Co 3d and O 2p. As the degree of Co 3d/O 2p orbital hybridization increases, a higher charge transfer was found between the active center and intermediate product, OOH, reducing the energy barrier of the rate-determining step while lowering the overpotential. This study provides thorough insight into the rational design of OER catalysts based on orbital hybridization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


