IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
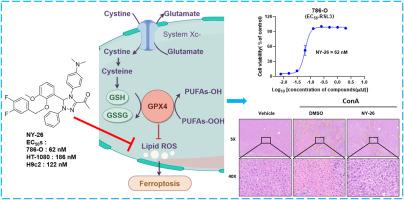
铁变态反应是一种新型的调节性细胞死亡形式,其特点是铁依赖性脂质 ROS 积累,与多种疾病相关,包括急性器官损伤、神经退行性疾病和癌症。药理抑制铁氧化酶具有治疗这些疾病的巨大潜力。然而,由于活性不足或药代动力学特征不理想,许多铁蛋白生成抑制剂的临床应用受到阻碍。在本研究中,通过对我们内部化合物库进行表型筛选,发现了几种 1,2,4-三唑衍生物可作为新型铁蛋白沉积抑制剂。在这些化合物中,NY-26 能在纳摩尔水平(EC50 = 62 nM)上显著抑制 RSL3 诱导的 786-O 细胞铁突变。NY-26 的抗铁细胞凋亡活性在多个细胞系中得到了进一步验证。机理研究表明,NY-26 是通过其固有的捕获自由基的抗氧化能力来抑制铁突变的。其他研究结果表明,在 ConA 诱导的急性肝损伤小鼠模型中,三唑衍生物可有效改善与铁突变相关的病理状况。综上所述,NY-26 与新型 1,2,4-三唑支架相连,可能是一种有效的铁蛋白沉积抑制剂,具有巨大的治疗潜力,有待进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery and optimization of 1,2,4-triazole derivatives as novel ferroptosis inhibitors
Ferroptosis is a novel form of regulated cell death characterized by iron-dependent lipid ROS accumulation, which is associated with various diseases, including acute organ injury, neurodegenerative disorders, and cancer. Pharmacological inhibition of ferroptosis has great potential for the treatment of these diseases. However, the clinical translation of many ferroptosis inhibitors is hindered by their inadequate activity or suboptimal pharmacokinetic profiles. In this study, several 1,2,4-triazole derivatives were identified as novel ferroptosis inhibitors through phenotypic screening of our in-house compound library. Among these compounds, NY-26 was found to significantly inhibit RSL3-induced ferroptosis in 786-O cells with nanomolar level (EC50 = 62 nM). The antiferroptotic activity of NY-26 was further validated across multiple cell lines. Mechanistic studies revealed that NY-26 inhibits ferroptosis through its intrinsic free radical-trapping antioxidant capacity. Additional results demonstrated that the triazole derivatives could effectively ameliorate ferroptosis-related pathological conditions in a mouse model of ConA-induced acute liver injury. Taken together, NY-26, tethering a novel 1,2,4-triazole scaffold, could be an effective ferroptosis inhibitor with great therapeutic potential for further investigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


