新型有机硒btsa衍生物作为有效的、可逆的、选择性的PPARγ共价调节剂用于抗糖尿病药物的发现
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
最近的研究发现,选择性过氧化物酶体增殖体激活受体γ (PPARγ)调节剂协同参与PPARγ- ser273磷酸化的抑制机制,是开发更安全、更有效的降糖药物的一种有希望的方法。在此,我们设计、合成和评价了一类新的有机硒化合物,即苯并噻唑-1-氧化物(BTSAs),作为有效的、可逆的和选择性的PPARγ共价调节剂。值得注意的是,2n,特别是(R)-2n,显示出高结合亲和力和优越的降糖作用,副作用减少。这主要是因为它能与PPARγ-LBD中的Cys285残基可逆地形成独特的共价键。进一步的机制研究表明,它主要通过有效抑制PPARγ-Ser273磷酸化、增强葡萄糖代谢和选择性上调胰岛素敏感基因的表达来表现出这种理想的药理学特征。总之,我们的研究结果表明(R)-2n有望成为治疗T2DM的先导化合物,并为未来的共价药物设计提供创新的可逆共价弹头参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
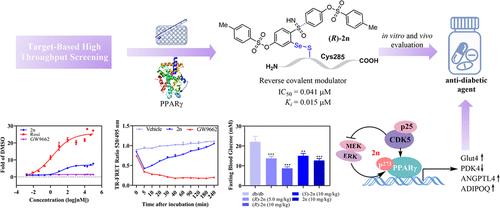
Identification of Novel Organo-Se BTSA-Based Derivatives as Potent, Reversible, and Selective PPARγ Covalent Modulators for Antidiabetic Drug Discovery
Recent studies have identified selective peroxisome proliferator-activated receptor γ (PPARγ) modulators, which synergistically engage in the inhibition mechanism of PPARγ-Ser273 phosphorylation, as a promising approach for developing safer and more effective antidiabetic drugs. Herein, we present the design, synthesis, and evaluation of a new class of organo-Se compounds, namely, benzothiaselenazole-1-oxides (BTSAs), acting as potent, reversible, and selective PPARγ covalent modulators. Notably, 2n, especially (R)-2n, displayed a high binding affinity and superior antidiabetic effects with diminished side effects. This is mainly because it can reversibly form a unique covalent bond with the Cys285 residue in PPARγ-LBD. Further mechanistic investigations revealed that it manifested such desired pharmacological profiles primarily by effectively suppressing PPARγ-Ser273 phosphorylation, enhancing glucose metabolism, and selectively upregulating the expression of insulin-sensitive genes. Collectively, our results suggest that (R)-2n holds promise as a lead compound for treating T2DM and also provides an innovative reversible covalent warhead reference for future covalent drug design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


