极简天然ORPphilin Macarangin B描述OSBP生物学功能
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
ORPphilin 家族的 OSBP 配体是化学性质复杂的天然产物,具有良好的抗癌特性。在这里,我们描述了一种对 OSBP 具有选择性的天然外消旋类黄酮--大黄素 B,它因其结构简单和独特的生物活性而从其他 ORPphilins 中脱颖而出。我们利用生物启发策略合成了(R,R,R)和(S,S,S)-macarangin B 对映体,从而能够根据它们独特的光学特性研究它们与 OSBP 的相互作用。实验和计算分析表明,(R,R,R)-macarangin B 与 OSBP 的亲和力最高。重要的是,与其他 ORPphilins 相比,这两种对映体的细胞毒性都明显降低,这表明 OSBP 并不是 ORPphilins 诱导细胞死亡的主要靶标。然而,OSBP 是一个有吸引力的抗病毒靶点,因为它被许多正链 RNA 病毒劫持。值得注意的是,(R,R,R)-macarangin B 能显著抑制寨卡病毒在人体细胞中的复制,突出了其作为抗病毒药物开发先导化合物的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
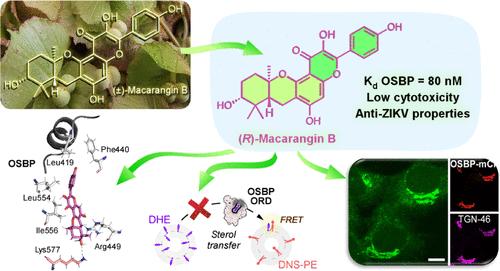
Minimalist Natural ORPphilin Macarangin B Delineates OSBP Biological Function
OSBP ligands from the ORPphilin family are chemically complex natural products with promising anticancer properties. Here, we describe macarangin B, a natural racemic flavonoid selective for OSBP, which stands out from other ORPphilins due to its structural simplicity and distinct biological activity. Using a bioinspired strategy, we synthesized both (R,R,R) and (S,S,S)-macarangin B enantiomers, enabling us to study their interaction with OSBP based on their unique optical properties. Experimental and computational analyzes revealed that (R,R,R)-macarangin B has the highest affinity for OSBP. Importantly, both enantiomers showed significantly decreased cytotoxicity compared to other ORPphilins, suggesting OSBP is not the primary target in ORPphilin-induced cell death. Yet, OSBP is an attractive antiviral target, as it is hijacked by many positive-strand RNA viruses. Remarkably, (R,R,R)-macarangin B significantly inhibited Zika virus replication in human cells, highlighting its potential as a lead compound for antiviral drug development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


