IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过酸促进 O-芳基羟胺的脱芳香重排,可得到 2-氨基环己二烯-1-酮,然后再对其进行还原淬灭,以合成以环己二烯为核心的反式氨基醇。这种方法是利用市售或容易制备的 2-芳基苯酚,通过两个步骤(一次纯化)合成 1-芳基环己胺支架的有效方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
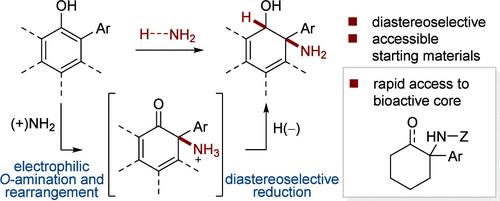
Formal Dearomative Hydroamination of 2-Arylphenols
An acid-promoted dearomative rearrangement of O-arylhydroxylamines affords 2-aminocyclohexadien-1-ones, which can in turn be reductively quenched for the synthesis of trans-aminoalcohols on a cyclohexadiene core. This method serves as an efficient entry to the pharmaceutically relevant 1-arylcyclohexylamine scaffold in two steps (one purification) from commercially available or readily prepared 2-arylphenols.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


