聚硫化物锂在二元导电聚环氧乙烷基共聚物电解质中的离子输运性质及加性效应
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
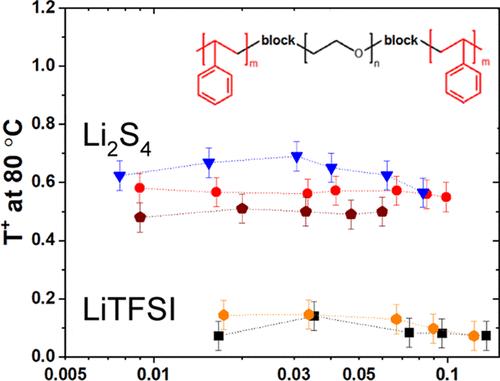
尽管锂硫(Li-S)电池可以带来比能量和电池寿命方面的高影响,但由于与该技术相关的固有问题,它们的全部优势尚未实现。放电过程中正极产生的中间多硫化物在电解液中溶解扩散,导致容量衰减,库仑效率低。这个问题的一个有希望的解决方案是使用固体聚合物电解质,它结合了离子导电聚环氧乙烷(PEO)相和机械增强相(如聚苯乙烯(PS))的优点,可以抑制锂在锂金属上的不均匀电沉积。在这项工作中,通过表征多硫化锂(Li2Sx, x = 4和8)的热力学、形态和离子传输性质,研究了使用PS-PEO-PS三嵌段共聚物作为Li-S电池电解质或粘合剂的可能性。热力学结果表明,与短链多硫化锂(Li2S4)相比,长链多硫化锂(Li2S8)更易于溶解于共聚物中。同时,在介结构嵌段共聚物中加入Li2S4和Li2S8会影响相转变(片层或六边形)和畴间距,其方式与传统的LiTFSI盐相似。在离子转移方面,与TFSI -阴离子相比,共聚物中多硫化物(S42 -和S82 -)的迁移率降低,阳离子转移数保持在0.5范围内,而LiTFSI为0.15。为了进一步应用,在LiTFSI盐存在的情况下,将Li2S4引入嵌段共聚物电解质中也用作添加剂,从而使锂金属电极的界面电阻非常低。这些研究结果将指导用于锂硫电池的固体聚合物电解质的设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ionic Transport Properties and Additive Effect of Lithium Polysulfides in Binary Conducting Poly(ethylene oxide)-Based Copolymer Electrolytes
Despite the high impact lithium–sulfur (Li–S) batteries can bring in terms of specific energy and battery lifetime, their full advantage has not yet been realized due to inherent issues associated with this technology. The intermediate polysulfide products produced in the positive electrode during discharge dissolve and diffuse in the electrolyte, leading to capacity fading and low Coulombic efficiency. A promising solution to this issue is the use of a solid polymer electrolyte that combines the advantages of an ion-conducting poly(ethylene oxide) (PEO) phase and a mechanically reinforced phase, such as polystyrene (PS), that can suppress the nonuniform electrodeposition of Li onto Li metal. In this work, the possibility of using PS–PEO–PS triblock copolymer as an electrolyte or binder in a Li–S battery was investigated by characterizing the thermodynamical, morphological, and ionic transport properties of lithium polysulfides species (Li2Sx, with x = 4 and 8). Thermodynamic results showed that the long-chain lithium polysulfide (Li2S8) is more soluble in the copolymers compared to the short-chain polysulfide (Li2S4). Meanwhile, the addition of Li2S4 and Li2S8 in the mesostructured block copolymer influences both the phase transition (lamellar or hexagonal) and the domain spacing in a fashion similar to the conventional LiTFSI salt. In terms of ionic transport, the mobility of the polysulfides (S42– and S82–) in the copolymers is reduced compared to the TFSI– anion, and the cationic transference number remains in the range of 0.5 compared to 0.15 for LiTFSI. To move toward the application, the introduction of Li2S4 into the block copolymer electrolyte is also used as an additive in the presence of LiTFSI salt, resulting in a very low interfacial resistance with the Li metal electrode. The results of these investigations would guide the design of solid polymer electrolytes for application in Li–S batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


