IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
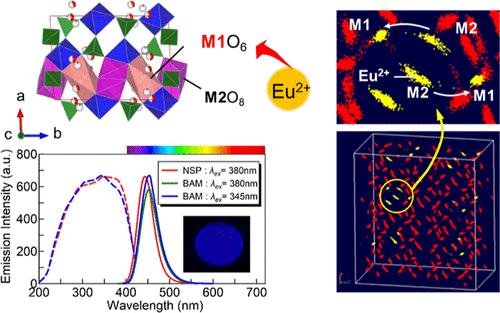
本研究旨在确定具有 NASICON 型框架的荧光粉在 Na3Sc2(PO4)3/Eu2+(NSP/Eu2+)主晶格中 Eu2+ 的位点分配。为此,我们进行了分子动力学(MD)模拟,其中采用了基于 NSP 多晶体晶体结构细化数据的绝热壳方法,并验证了该方法的有效性。对优质单晶体的精确晶体结构分析表明存在三种类型的物相:一种是之前报道过的 R3̅c 空间群的γ 物相[γ(trig)-NSP],一种是 I2/a 空间群的单斜物相[α(mono)-NSP],还有一种是 C2/c 空间群的单斜物相[γ(mono)-NSP]。在具有两个晶体学上独立的 Na 位点的 α(单)-NSP 的 MD 模拟中,Na+ 离子经常在两个位点之间跳跃。然而,对一种 Na+ 离子部分被 Eu2+ 离子和空位取代的晶胞进行的 MD 模拟表明,Eu2+ 离子优先位于畸变的八面体位点,Na+ 离子跳变没有发生。通过传统固态反应方法得到的α(单)-NSP相掺杂Eu2+的荧光粉在370 nm波长的照射下发出强烈的蓝色荧光,这种荧光属于Eu2+ d-f转变,而(三)相荧光粉发出的光强度较低。当 K+ 离子取代 Na+ 离子位点时,α(单)-NSP 相荧光粉的发光和热淬灭性能得到改善。与 NSP/Eu2+ 相比,量子产率明显提高,几乎可与商用 BaMgAl10O17/Eu2+ (BAM)荧光粉相媲美。根据晶体结构细化和 MD 模拟结果讨论了 NSP/Eu2+ 的发光特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Polymorphs of NASICON-Type Na3Sc2(PO4)3/Eu2+ Phosphors Analyzed by Single Crystal Structure Determination and Molecular Dynamics Simulations
This study aimed to determine the site assignment of Eu2+ in the Na3Sc2(PO4)3/Eu2+ (NSP/Eu2+) host lattice for phosphors with NASICON-type frameworks. For this purpose, molecular dynamics (MD) simulations, in which an adiabatic shell method based on crystal structure refinement data for polymorphs of NSP, was employed and verified to be effective. Precise crystal structure analysis of good-quality single crystals indicated the presence of three types of phases: a γ phase assigned to the R3̅c space group [γ(trig)-NSP] reported previously, a monoclinic phase assigned to the I2/a space group [α(mono)-NSP], and another monoclinic phase assigned to the C2/c space group [γ(mono)-NSP]. In the MD simulations of α(mono)-NSP with two crystallographically independent Na sites, Na+ ion hopping between the sites frequently occurred. However, the MD simulations of the cells with one type of Na+ ion partially replaced by an Eu2+ ion and vacancy showed that the Eu2+ ions were preferentially located at a distorted octahedral site, and Na+ ion hopping did not occur. The α(mono)-NSP-phase Eu2+-doped phosphors obtained via a conventional solid-state reaction method exhibited intense blue luminescence, which was assigned to the Eu2+ d–f transition, under irradiation at 370 nm, whereas the intensity of the light emitted by the (trig)-phase phosphors was lower. The luminescence and thermal quenching of the α(mono)-NSP phase phosphors was improved when K+ ions were substituted at Na+ ion sites. The quantum yields were significantly improved compared to those of NSP/Eu2+, being almost comparable with those of a commercial BaMgAl10O17/Eu2+ (BAM) phosphor. The luminescence properties of NSP/Eu2+ are discussed based on the crystal structure refinement and MD simulation results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


