C-S-H孔中的空化、亲水性和吸附滞后:相对湿度和温度的耦合效应
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
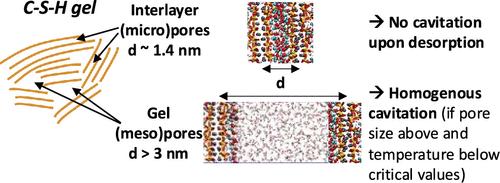
吸附过程对于水泥基材料的干燥和耐久性至关重要,会直接影响其热性能。温度会对这些过程产生重大影响。本研究利用分子模拟来研究不同温度和相对湿度条件下 C-S-H 孔隙中的吸附作用,考虑了从凝胶到层间尺度(11.6 到 106 Å)的孔隙大小。我们量化了 C-S-H 孔隙中水空化和吸附滞后与温度和孔隙大小的关系。我们确定了滞后消失和毛细管凝结可逆的临界孔隙尺寸,前者与空化直接相关。我们发现,只有当凝胶(中)孔高于临界孔径并低于空化临界温度时,才会发生空化。层间孔隙是 C-S-H 中的一类主要微孔,不会发生空化现象。C-S-H 孔隙中的空化是均匀的,发生在中孔的块状区域。C-S-H 表面的亲水性随着温度的升高而增加,使得异质空化不太可能发生。上述结果是通过三种不同的力场参数化一致得出的,这使我们对其描述 C-S-H 界面行为的相关性更有信心。最后,我们证明了孔隙排空和填充的宏观考虑因素,如开尔文-科汉方程和 Derjaguin-Broekhoff-de Boer 平衡方程,在 C-S-H 中通过空化发生解吸时是无效或不准确的。这些结果有助于理解其他纳米层吸附材料的吸附过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cavitation, Hydrophilicity, and Sorption Hysteresis in C–S–H Pores: Coupled Effects of Relative Humidity and Temperature
Sorption processes are critical for the drying and durability of cement-based materials, directly affecting their thermal properties. Temperature can substantially influence these processes. This work uses molecular simulations to study sorption in C–S–H pores under varying temperatures and relative humidity, considering pore sizes from the gel to the interlayer scale (between 11.6 and 106 Å). We quantify the temperature and pore-size dependence of water cavitation and sorption hysteresis in the C–S–H pores. The critical pore sizes for the disappearance of hysteresis and the reversibility of capillary condensation are identified, with the former being directly associated with cavitation. We show that cavitation occurs only in gel (meso)pores when they are above the critical pore size and below the critical temperature for cavitation. Interlayer pores, a major class of micropores in C–S–H, are not subjected to cavitation. Cavitation in C–S–H pores is homogeneous, occurring in the bulk-like zone of mesopores. The hydrophilicity of the C–S–H surface increases with the temperature, making heterogeneous cavitation less likely to occur. The results above were obtained consistently with three different force field parametrizations, building confidence in their relevance to describe C–S–H interfacial behavior. Finally, we demonstrate that macroscopic considerations for pore emptying and filling, such as the Kelvin-Cohan and equilibrium Derjaguin-Broekhoff-de Boer equations, are not valid or inaccurate when desorption occurs through cavitation in C–S–H. These results are relevant to understanding the sorption processes in other nanolayered adsorbing materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


