低温电镜观察丹trolene对携带严重恶性高热突变Y522S的ryanodine受体1的抑制作用
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
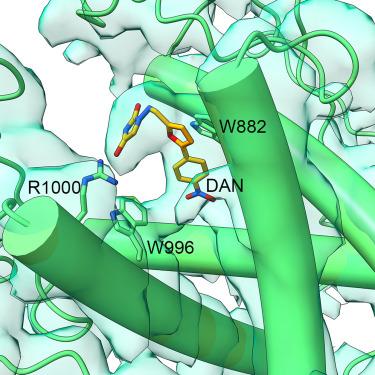
ryanodine受体1 (RyR1)的骨骼异构体突变在麻醉或琥珀胆碱肌肉松弛剂治疗期间会造成严重风险。这些可通过主要由RyR1通道渗漏引起的细胞内钙增加引发潜在致命的恶性高热(MH)发作。丹trolene是唯一已知的预防死亡的治疗选择。丹trolene的主要靶点是RyR1;然而,人们对其抑制机制知之甚少。在2.5-3.3 Å分辨率下,与严重MH Y522S RyR1突变体结合的丹trolene在关闭和打开状态下的冷冻电镜(cro - em)结构显示,该药物结合到远离离子门的通道细胞质组装上,与P1结构域的残基W882、W996和R1000相互作用。这一发现得到了野生型(WT)和丙氨酸突变体Ca2+成像和[3H]ryanodine结合的验证。丹trolene通过限制中心激活模块,“冷却”由突变引起的启动构象来降低通道打开概率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dantrolene inhibition of ryanodine receptor 1 carrying the severe malignant hyperthermia mutation Y522S visualized by cryo-EM
Mutations in the skeletal isoform of the ryanodine receptor 1 (RyR1) pose grave risks during anesthesia or treatment with succinylcholine muscle relaxants. These can trigger a potentially lethal malignant hyperthermia (MH) episode via intracellular calcium increase mainly from RyR1 channel leakage. Dantrolene is the only known treatment option to prevent death. The main target of dantrolene is RyR1; however, little is known about the mechanism of inhibition. Cryoelectron microscopy (cryo-EM) structures of dantrolene bound to the severe MH Y522S RyR1 mutant in the closed and open states at 2.5–3.3 Å resolution revealed that the drug binds to the channel’s cytoplasmic assembly, far from the ion gate, interacting with residues W882, W996, and R1000 in the P1 domain. The finding was validated by Ca2+ imaging and [3H]ryanodine binding in wild-type (WT) and alanine mutants. Dantrolene reduced channel opening probability by restricting the central activation module, “cooling down” the primed conformation caused by the mutation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


