镁酚纳米编辑器精炼胶质瘤T细胞用于金属免疫治疗
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
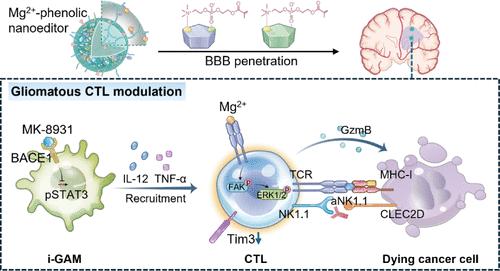
与胶质母细胞瘤的稀疏浸润相比,细胞毒性T淋巴细胞(ctl)的功能也低下,并过度表达抑制标志物,特别是已鉴定的NK细胞受体(NK1.1)。然而,大多数研究只关注如何增强肿瘤浸润性ctl,而忽视了其杀伤维持。金属免疫疗法已被证明可以改善ctl的功能,但由于安全输送和大脑生理特性的严重限制,它几乎无法适应胶质母细胞瘤。在此,我们合成了一种两亲性聚乙二醇(PEG)聚合物(指定为MPP),该聚合物由胆碱类似物2-甲基丙烯酰氧乙基磷酸胆碱(MPC)和多酚基团修饰,通过配合Mg2+并包裹疏水性BACE1抑制剂MK-8931来定制纳米编辑器(Mg2+@MK-8931@MPP),然后精确地纠正胶质瘤CTL稀疏和细胞毒性功能障碍。Mg2+@MK-8931@MPP纳米编辑器在mpc辅助的胶质母细胞瘤局部积聚后,释放MK-8931使m2样巨噬细胞再极化,定量促进CTL浸润。新生免疫佐剂Mg2+进一步强化t细胞受体下游信号,在质量上增强进入ctl的功能,导致高水平抗肿瘤细胞因子和细胞毒蛋白的分泌。通过抗NK1.1抗体进一步阻断NK1.1对ctl的抑制作用,可以延长其细胞溶解的终局时间。对t细胞缺陷和野生型小鼠模型的研究支持Mg2+@MK-8931@MPP的免疫调节可行性。这种胶质瘤ctl量身定制的策略同时拓宽了金属免疫治疗到胶质母细胞瘤的治疗,并强调了加强胶质瘤ctl功能的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Magnesium–Phenolic Nanoeditor Refining Gliomatous T Cells for Metalloimmunotherapy
More than the sparse infiltration in glioblastoma, cytotoxic T lymphocytes (CTLs) also function inefficiently and overexpress the inhibitory markers, especially the identified NK cell receptor (NK1.1). However, most studies solely focus on how to augment tumor-infiltrating CTLs and overlook their killing maintenance. Metalloimmunotherapy has been proven to improve the functionalities of CTLs, but it has barely adapted to glioblastoma due to the severe limitations of safe delivery and the brain’s physiological properties. Herein, we synthesized an amphipathic polyethylene glycol (PEG) polymer (designated as MPP) modified with the choline analogue 2-methacryloyloxyethyl phosphorylcholine (MPC) and polyphenol moieties to customize a nanoeditor (Mg2+@MK-8931@MPP) by coordinating Mg2+ and entrapping the hydrophobic BACE1 inhibitor MK-8931, then precisely redressing the gliomatous CTL sparsity and cytotoxic dysfunction. Upon MPC-assisted local accumulation in glioblastoma, Mg2+@MK-8931@MPP nanoeditors release MK-8931 to repolarize M2-like macrophages, facilitating CTL infiltration quantitatively. The cenogenetic immune adjuvant Mg2+ ulteriorly fortifies the T-cell receptor downstream signals to enhance the functionality of the ingoing CTLs in quality, leading to the secretion of high-level antitumor cytokines and cytotoxic proteins. Further blocking the inhibitory NK1.1 on CTLs by anti-NK1.1 antibodies can extend their cytolytic endgame. Studies on T-cell-deficient and wild-type mouse models support the immunomodulating feasibility of Mg2+@MK-8931@MPP. This gliomatous CTL-tailored strategy concurrently broadens metalloimmunotherapy to glioblastoma treatment and highlights the necessity of enforcing gliomatous CTLs’ functionality.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


