可见光介导炔烃与NHC硼烷的自由基反式硼化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
虽然烯烃与n -杂环碳烷(NHC)硼烷的自由基硼化反应有很好的文献记载,但炔的自由基硼化反应,特别是末端炔的自由基硼化反应仍然具有挑战性。本文研究了一种光氧化还原催化烷基与NHC硼烷的自由基反式硼氢化反应,得到了多种产率中高的烯基硼化合物。该方案具有广泛的底物范围,因为内炔和端炔都是相容的。应用于生物活性分子的后期修饰,进一步证明了该方法的合成价值。提出了该反应的初步机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
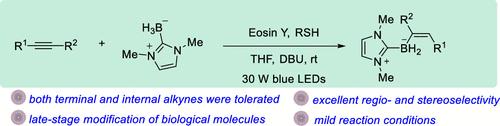
Visible-Light-Mediated Radical trans-Hydroboration of Alkynes with NHC Borane
Although the radical hydroboration of alkenes with N-heterocyclic carbene (NHC) borane is well documented, the radical hydroboration of alkynes, especially terminal alkynes, remains challenging. Herein, a photoredox-catalyzed radical trans-hydroboration of alkynes with NHC borane has been developed, which provided various alkenyl boron compounds in moderate to good yields. This protocol exhibits a broad substrate scope, as both internal and terminal alkynes were compatible. The synthetic value of this method was further demonstrated by its applicability in the late-stage modification of bioactive molecules. A preliminary mechanism for this reaction was proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


