n-烷基碳卟啉-2-碳醛类卟啉的合成、反应性和芳香特性:镍(II)和钯(II)配合物、弱异位性21-氧碳卟啉和强芳香性三氧碳卟啉的分离
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
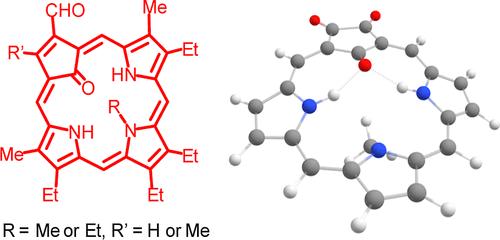
用酸催化n-烷基三吡喃与1,3-环戊二烯或甲基-1,3-环戊二烯衍生的三醛缩合,然后在氯化铁水溶液中氧化,得到收率为22-27%的23-烷基-21-碳卟啉-2-碳乙醛和弱芳氧碳卟啉。碳卟啉与醋酸钯(II)或醋酸镍(II)反应生成有机金属配合物,但在这两种情况下都发生烷基迁移,生成21烷基衍生物。虽然这种类型的反应性以前已经在钯配合物中观察到,但这是第一次在镍(II)碳卟啉中看到这种现象。在回流DMF中与醋酸镍反应较长时间主要导致分解,但发现了不寻常的副产物。在3级氧化铝的存在下,碳卟啉醛与5等量的(二(三氟乙酰氧基)碘)苯反应,可以得到更高的收率。脱碳和氧化产生嵌入在类卟啉大环中的三氧环戊烷片段。从质子核磁共振谱、核无关化学位移(NICS)计算和诱导环电流图的各向异性可以看出,新体系具有强烈的芳香性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, Reactivity and Aromatic Characteristics of Porphyrinoids Derived from N-Alkylcarbaporphyrin-2-Carbaldehydes: Isolation of Nickel(II) and Palladium(II) Complexes, Weakly Diatropic 21-Oxycarbaporphyrins and Strongly Aromatic Trioxycarbaporphyrins
Acid catalyzed condensation of N-alkyltripyrranes with trialdehydes derived from 1,3-cyclopentadiene or methyl-1,3-cyclopentadiene, followed by oxidation with aqueous ferric chloride solutions, gave 23-alkyl-21-carbaporphyrin-2-carbaldehydes in 22–27% yield together with weakly aromatic oxycarbaporphyrins. The carbaporphyrins reacted with palladium(II) acetate or nickel(II) acetate to give organometallic complexes but in both cases alkyl group migration took place to generate 21-alkyl derivatives. Although this type of reactivity had been observed previously for palladium complexes, this is the first time the phenomenon has been seen in nickel(II) carbaporphyrins. Reactions with nickel(II) acetate in refluxing DMF for longer time periods primarily led to decomposition but unusual byproducts were identified. These could be obtained in higher yields by reacting the carbaporphyrin aldehydes with 5 equiv of (bis(trifluoroacetoxy)iodo)benzene in the presence of grade 3 alumina. Decarbonylation and oxidation generate a trioxocyclopentane moiety that is embedded in the porphyrinoid macrocycle. The new system has strongly aromatic properties that are evident from the proton NMR spectra, nucleus independent chemical shift (NICS) calculations and anisotropy of induced ring current plots.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


