高性能氮化镍铁燃料电池和电解槽电催化剂的表面调制见解
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
催化对能源转换技术的发展和商业化至关重要。必须找到丰富、活跃和稳定的材料,才能在燃料电池(FC)和电解槽等可再生技术中可靠、经济地使用催化剂。在第一排过渡金属中可以找到合适的候选材料,如非贵金属,在这些金属中可以很容易地合成双金属、金属氧化物和金属氮化物等材料。最近,这些材料在碱性介质中的氧还原(ORR)和氧进化(OER)反应中表现出很高的活性,这反过来又与 FC 和电解槽中的良好性能有关。然而,这些研究大多没有超出半电池反应的范围。在本研究中,我们探讨了金属氮化物 Ni3FeN 的合成及其作为 ORR 和 OER 电催化剂的应用。我们开发了使用一步氨解路线合成不同碳负载的 Ni3FeN 纳米晶体的程序。我们的研究表明,该材料的原始结构包括氮核和厚度为几纳米的氧化物外壳。然而,主体电子结构主要由 Ni3FeN 相主导。氮化物在 1 M KOH 中表现出令人印象深刻的稳定 ORR 性能,有利于 4 e- 途径。在长时间(100 K)的加速应力测试(AST)中,该材料的 E1/2 值略微下降了 10 mV(与 RHE 相比,从 0.85 V 降至 0.84 V)。AST 在 ORR 电位下的降解表明催化剂聚集成了较大的纳米颗粒,形成了 Ni@NiFeOx 结构。在 OER 电位下进行测试后,催化剂破碎成较小的纳米颗粒,并主要倾向于 NiFeOx 结构。在以氢气为燃料的碱性交换膜燃料电池(AEMFC)中对 Ni3FeN ORR 催化剂进行了 MEA 测试,结果表明其峰值功率密度约为 700 mW/cm2,是氮化物和镍铁合金材料中最高的。我们相信,这项工作能使镍钴基材料成为燃料电池应用中可行的廉价替代品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
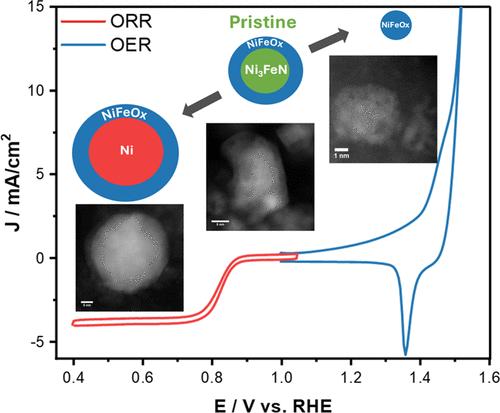
Surface Modulation Insights of High-Performing Ni–Fe Nitride Fuel Cell and Electrolyzer Electrocatalysts
Catalysis has been crucial in advancing and commercializing energy conversion technologies. It is essential to identify abundant, active, and stable materials to enable the reliable and cost-efficient use of catalysts in renewable technologies, such as fuel cells (FCs) and electrolyzers. Suitable candidates, such as nonprecious metals, can be found in first-row transition metals, where materials such as bimetallics, metal oxides, and metal nitrides can be readily synthesized. Recently, these materials have exhibited high activity toward the oxygen reduction (ORR) and oxygen evolution (OER) reactions in alkaline media, which, in turn, were related to promising performance in FCs and electrolyzers. However, most of these studies have not gone beyond half-cell reactions. In this study, we explored the synthesis of a metal nitride, Ni3FeN, and its application as an electrocatalyst for ORR and OER. We developed procedures for the synthesis of Ni3FeN nanocrystals with different carbon loadings using a one-step ammonolysis route. We show that the pristine structure of the material encompasses a nitride core and an oxide shell with a thickness of a few nanometers. However, the bulk electronic structure is mainly dominated by the Ni3FeN phase. The nitride exhibited an impressive and stable ORR performance in 1 M KOH favoring the 4 e– pathway. The material exhibited a slight decrease in E1/2 of 10 mV (from 0.85 to 0.84 V vs RHE) during a prolonged (100 K) accelerated stress test (AST). The AST degradation at ORR potentials indicates that the catalyst aggregates into larger nanoparticles, forming a Ni@NiFeOx structure. After tests at OER potentials, the catalyst breaks into smaller nanoparticles and mainly favors the NiFeOx structure. MEA testing of the Ni3FeN ORR catalyst in a hydrogen-fueled alkaline exchange membrane fuel cell (AEMFC) yielded a peak power density of ca. 700 mW/cm2; among the highest reported for nitride and NiFe-based materials. We believe that this work could enable the use of NiFe-based materials as viable, inexpensive alternatives for fuel cell applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


