转铁蛋白电晕靶向递送替拉帕扎胺和 IR820,促进对 4T1 乳腺癌进行高效的光诱导缺氧化疗
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
蛋白冠(PC)的形成赋予了原始纳米材料新的生物学特性,阻碍了其在细胞和组织中的吸收和靶向作用。尽管许多讨论PC形成的研究都集中在可能抑制纳米材料功能的惰性蛋白上,但一些具有内在靶向能力的功能性血浆蛋白也可以吸附在纳米材料表面,具有活性配体性质,以提高靶向能力。在这种方法中,纳米材料经过表面工程处理,以促进直接靶向的特定功能血浆蛋白的吸附,从而将纳米材料运输到靶点。本研究利用T10肽修饰脂质体构建了原位转铁蛋白(Tf) pc介导的脂质体,该脂质体携带缺氧敏感化疗药物(替拉帕胺,TPZ)和光敏剂(吲哚菁绿,IR820)。将水溶性药物TPZ包封在介孔二氧化硅纳米颗粒(MSNs)中,并包被负载IR820 (IR)的脂质体。脂质包被的msn可以抑制体内聚集,显著降低水溶性药物的快速释放,从而提高系统稳定性和缓释。T10进入体内循环后,与血浆中的Tf特异性结合,形成原位Tf脂质体- pc复合物,与传统的配体修饰的主动靶向策略相比,靶向效果增强。然而,大尺寸PC颗粒在深入肿瘤组织方面面临挑战。IR可以通过光动力疗法(PDT)杀死肿瘤,并与低氧敏感药物TPZ互补抗肿瘤。这项研究证明了原位pc介导的多功能脂质体用于缺氧激活化疗联合PDT的新设计,这是一种很有前途的癌症治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
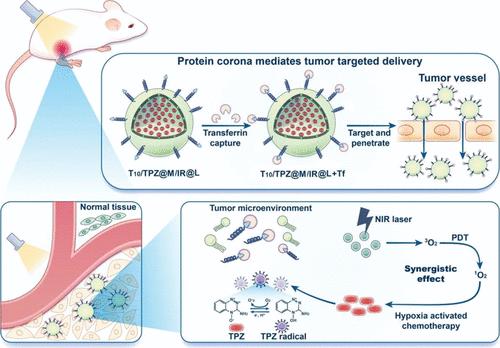
Transferrin Protein Corona-Targeted Codelivery of Tirapazamine and IR820 Facilitates Efficient PDT-Induced Hypoxic Chemotherapy on 4T1 Breast Cancer
Protein corona (PC) formation confers novel biological properties to the original nanomaterial, impeding its uptake and targeting efficacy in cells and tissues. Although many studies discussing PC formation have focused on inert proteins that may inhibit the function of nanomaterials, some functional plasma proteins with intrinsic targeting capabilities can also be adsorbed to the surface of nanomaterials, with active ligand properties to improve the targeting ability. In this approach, nanomaterials are surface-engineered to promote the adsorption of specific functional plasma proteins that are directly targeted to transport nanomaterials to the target site. In this study, T10 peptide-modified liposomes were employed to construct an in situ transferrin (Tf) PC-mediated liposome carrying a hypoxia-sensitive chemotherapy drug (tirapazamine, TPZ) and a photosensitizer (indocyanine green, IR820). The water-soluble drug TPZ was encapsulated in mesoporous silica nanoparticles (MSNs) and coated with IR820 (IR)-loaded liposome. Lipid-coated MSNs can inhibit aggregation in the body and significantly reduce the rapid release of water-soluble drugs, resulting in improved system stability and sustained release. Upon entering the in vivo circulation, T10 bound specifically to Tf in plasma to form an in situ Tf liposome–PC complex with enhanced targeting efficacy compared to traditional ligand-modified active-targeting strategies. However, large-sized PC particles faced challenges in penetrating deep into tumor tissues. IR could kill tumors through photodynamic therapy (PDT) and elicit complementary antitumor effects with the hypoxia-sensitive drug TPZ. This study demonstrates the novel design of in situ PC-mediated multifunctional liposomes for hypoxia-activated chemotherapy combined with PDT, a promising approach to cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


