基于酰腙改性甲酰聚苯乙烯的酰基酰腙复合材料的快速合成及其对水中重金属离子和硫化物的去除效果
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
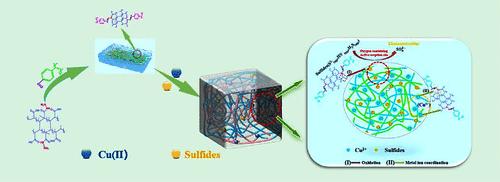
本研究对废聚苯乙烯进行改性升级制备甲酰化聚苯乙烯,并与聚甲基丙酰肼缩合合成改性聚苯乙烯乙酰腙(LT-HPA)。通过各种表征方法证明对吸附材料的改性是成功的。在随后的污染物去除研究中,考察了pH、质量、浓度、接触时间和盐离子干扰。去除Cu(II)和硫化物的最佳pH分别为5和11,吸附剂的最佳质量为1 g/L。结果表明,该工艺对Cu (II)的最大去除率为231.51 mg/g。吸附平衡时间小于40 min,这是由于骨架上酰基酰肼基团通过配位对Cu (II)具有良好的吸附活性。60 min最大脱硫量为370.21 mg/g,主要是硫化物被表面氧化基团氧化所致。等温线和动力学研究表明,Cu (II)和硫化物的吸附过程符合Langmuir模型和拟二级动力学模型。热力学参数表明,Cu (II)和硫化物的脱除是一个自发吸热过程。吸附机理以化学吸附为主,物理吸附为辅。经过10次再生吸附/脱附循环后,Cu (II)和硫化物的去除率保持在85%以上,表明聚苯乙烯磺酸乙酯(LT-HPA)吸附材料具有良好的可重复利用性。此外,吸附剂具有良好的热稳定性、耐盐性和环境友好性。综上所述,改性甲酰化聚苯乙烯乙酰腙(LT-HPA)在脱除铜(II)和硫化物方面具有良好的应用前景,为聚苯乙烯的升级换代提供了一条新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Facile Synthesis of Acyl-Hydrazone Composites Based on Hydrazide-Modified Formylated Polystyrene for Effective Removal of Heavy Metal Ions and Sulfides from Water
In this study, waste polystyrene was modified and upgraded to prepare formylated polystyrene, and the modified polystyrene acetyl hydrazone (LT-HPA) was synthesized by condensation with polymethyl-propionyl-hydrazine. It is proven that the modification of the adsorption material is successful by various characterization methods. In the subsequent pollutant removal study, pH, mass, concentration, contact time, and salt ion interference were investigated. The optimal pH for Cu(II) and sulfide removal was 5 and 11, respectively, and the optimal mass of adsorbent was 1 g/L. It was found that the maximum removal rate of Cu (II) was 231.51 mg/g. The adsorption equilibrium time was less than 40 min. This is due to the good adsorption activity of acyl hydrazide groups on the skeleton for Cu (II) through coordination. The maximum desulfurization amount in 60 min was 370.21 mg/g, which was mainly caused by the oxidation of sulfides by surface oxidizing groups. Isotherm and kinetic studies show that the adsorption processes of Cu (II) and sulfides are consistent with the Langmuir model and pseudo-second-order kinetic model. Thermodynamic parameters show that the removal of Cu (II) and sulfides is a spontaneous endothermic process. The adsorption mechanism is mainly chemical adsorption, supplemented by physical adsorption. After 10 regenerated adsorption/desorption cycles, the removal rates of Cu (II) and sulfides remained above 85%, indicating that polystyrenesulfonate acetylhydrazone (LT-HPA) adsorption materials have good reusability. In addition, the adsorbent has good thermal stability, salt resistance, and environmental friendliness. In summary, the modified formylated polystyrene acetyl hydrazone (LT-HPA) has a good application prospect in the removal of copper(II) and sulfides and provides a fresh way for upgrading polystyrene.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


