分子取向的作用:氮芳小分子抑制剂在区域选择性原子层沉积中的比较
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
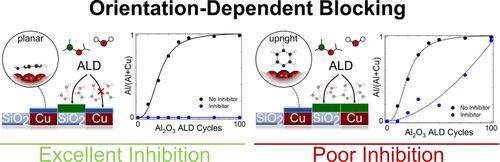
区域选择性原子层沉积(AS-ALD)在满足半导体工业对精密纳米图形的严格要求方面显示出巨大的潜力。小分子抑制剂(SMIs)最近被证明是一种有前途的、工业兼容的实现AS-ALD的方法。在这项工作中,我们比较了三种含氮芳香SMIs -苯胺,吡咯和吡啶-在有(CuOx)和没有(Cu)天然氧化物的铜上阻断Al2O3 ALD的能力。我们发现吡咯和苯胺作为抑制剂的性能比吡啶好得多。此外,在ABC模式下,每次ALD循环前,吡咯和苯胺都能在铜表面上进行抑制,促进了在Cu存在的情况下,超过11 nm的Al2O3在SiO2生长表面上的选择性沉积,选择性为99.9%。通过理论和实验的结合,我们对选择性延长和丧失的机制提供了新的认识。首先,我们发现吡咯和苯胺以平面键取向吸附,而吡啶在铜表面垂直结合,我们提出直立分子取向是吡啶无效抑制的起源。其次,我们发现CuOx表面固有地比Cu表面更具反应性,导致最终失去选择性,尽管再给药抑制剂。最后,我们观察到苯胺的重加保护铜表面免受不希望的氧化,而吡啶的重加则没有。因此,我们假设再给药的一个可能的好处是防止氧化,从而减少ALD期间活性位点的形成。通过这项工作,我们证明了氮芳烃作为as -ALD的SMIs的能力,我们对分子取向在抑制中的作用以及ALD工艺参数对选择性的影响提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Role of Molecular Orientation: Comparison of Nitrogenous Aromatic Small Molecule Inhibitors for Area-Selective Atomic Layer Deposition
Area-selective atomic layer deposition (AS-ALD) shows great potential for meeting the stringent demands of the semiconductor industry for precision nanopatterning. Small molecule inhibitors (SMIs) have recently proven to be a promising, industry-compatible means of achieving AS-ALD. In this work, we compare three nitrogenous aromatic SMIs – aniline, pyrrole, and pyridine – for their ability to block Al2O3 ALD on copper with (CuOx) and without (Cu) a native oxide. We find that pyrrole and aniline perform much better as inhibitors than pyridine does. Furthermore, when redosed on copper before every ALD cycle in an ABC scheme, pyrrole and aniline provide outstanding inhibition, facilitating the selective deposition of over 11 nm of Al2O3 on an SiO2 growth surface in the presence of Cu with 99.9% selectivity. By combining both theory and experiment, we provide new understanding of the mechanisms by which selectivity is prolonged and lost. First, we show that whereas pyrrole and aniline adsorb in a planar bonding orientation, pyridine binds upright at the copper surface, and we propose that the upright molecular orientation is the origin of the ineffective inhibition of pyridine. Second, we find that the CuOx surface is inherently more reactive than the Cu surface, leading to an eventual loss of selectivity, despite the redosing of the inhibitor. Finally, we observe that redosing of aniline protects the copper surface from undesired oxidation, whereas the redosing of pyridine does not. As such, we posit that a likely benefit of redosing is preventing oxidation and thus reducing reactive site formation during ALD. Through this work, we demonstrate the capability of nitrogenous aromatics to serve as SMIs for AS-ALD, and we contribute insights regarding the role of molecular orientation on inhibition and the impact of ALD process parameters on selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


